Abstract
Rotenone, a mitochondrial complex I inhibitor, can induce the pathological features of Parkinson's disease (PD). In the present study, naringin, a grapefruit flavonoid, inhibited rotenone-induced cell death in human neuroblastoma SH-SY5Y cells. We assessed cell death and apoptosis by measuring mitogen-activated protein kinase (MAPKs) and caspase (CASPs) activities and by performing 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay, 4,6-diamidino-2-phenylindole (DAPI) staining, and terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) staining. Naringin also blocked rotenone- induced phosphorylation of Jun NH2 – term inal protein kinase (JNK) and P38, and prevented changes in B-cell CLL/lymphoma 2 (BCL2) and BCL2-associated × protein (BAX) expression levels. In addition, naringin reduced the enzyme activity of caspase 3 and cleavages of caspase 9, poly (ADP-ribose) polymerase (PARP), and caspase 3. These results suggest that naringin has a neuroprotective effect on rotenone-induced cell death in human neuroblastoma SH-SY5Y cells.
Go to : 
REFERENCES
Bradshaw J., Saling M., Hopwood M., Anderson V., Brodtmann A. Fluctuating cognition in dementia with Lewy bodies and Alzheimer's disease is qualitatively distinct. J Neurol Neurosurg Psychiatry. 75:382–387. 2004.

Chang L., Karin M. Mammalian MAP kinase signaling cascades. Nature. 410:37–40. 2000.
Dudley DT., Pang L., Decker SJ., Bridged AJ., Saltiel AR. A synthetic inhibitor of the mitogen-activated protein kinase cascade. Proc Natl Acad Sci USA. 92:7686–7689. 1995.

Greenamyre JT., Sherer TB., Betarbet R., Panov AV. Complex I and Parkinson's disease. IUBMB Life. 52:135–141. 2001.

Haenen GR., Paquay J., Korthouwer R., Bast A. Peroxynitrite scavenging by flavonoids. Biochem Biophys Res Commun. 236:591–593. 1997.

Junn E., Mouradian MM. Apoptotic signaling in dopamine induced cell death: the role of oxidative stress, P38 mitogen-activated protein kinase, cytochrome c and caspases. J Neurochem. 78:374–383. 2001.
Kanno S., Shouji A., Asou K., Ishikawa M. Effects of naringin on hydrogen peroxide-induced cytotoxicity and apoptosis in P388 cells. J Pharmacol Sci. 92:166–170. 2003.

Kaul TN., Middlenton E Jr., Ogra PL. Antiviral effect of flavonoids on human viruses. J Med Virol. 15:71–79. 1985.

Klintworth H., Newhouse K., Li T., Choi WS., Faigle R., Xia Z. Activation of c-Jun N-terminal protein kinase is a common mechanism underlying paraquat- and rotenone-induced dopaminergic cell apoptosis. Toxicol Sci. 97:149–162. 2007.

Li P., Nijhawan D., Budihardjo I., Srinivasula SM., Ahmad M., Alnemri ES., Wang X. Cytochrome c and dATP dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 91:479–489. 2004.
Maridonneau-Parini I., Braquet P., Garay RP. Heterogeneous effect of flavonoids on K+-loss and lipid peroxidation induced by oxygen free radicals in human red cells. Pharmacol Res Commun. 18:61–72. 1986.
Newhouse K., Hsuan SL., Chang SH., Cai B., Wang Y., Xia Z. Rotenone-induced apoptosis is mediated by p38 and JNK MAP kinases in human dopaminergic SH-SY5Y cells. Toxicol Sci. 79:137–146. 2004.

Ng TB., Liu F., Wang ZT. Antioxidative activity of natural products from plants. Life Sci. 66:709–723. 2000.

Olanow CW., Perl DP., DeMartino GN., McNaught KS. Lewy-body formation is an aggresome-related process: a hypothesis. Lancet Neurol. 3:496–503. 2004.

Park HJ., Shin DH., Chung WJ., Leem K., Yoon SH., Hong MS., Chung JH., Bae JH., Hwang JS. Epigallocatechin gallate reduces hypoxia-induced apoptosis in human hepatoma cells. Life Sci. 78:2826–2832. 2006.

Pei W., Liou AK., Chen J. Two caspase-mediated apoptotic pathways induced by rotenone toxicity in cortical neuronal cells. FASEB J. 17:520–522. 2003.

Ramsey CP., Giasson BI. Role of mitochondrial dysfunction in Parkinson's disease-implications for treatment. Drugs Aging. 24:95–105. 2007.
Rundén E., Seglen PO., Haug FM., Ottersen OP., Wieloch T., Shamloo M., Laake JH. Regional selective neuronal degeneration after protein phosphatase inhibition in hippocampal slice cultures: evidence for a MAP kinase-dependent mechanism. J Neurosci. 18:7296–7305. 1998.

Saporito MS., Brown EM., Miller M S., Carswell S. CEP-1347/KT-7515, an inhibitor of c-jun N-terminal kinase activation, attenuates the 1-methyl-4-phenyl tetrahydropyridine-mediated loss of nigrostriatal dopaminergic neurons in vivo. J Pharmacol Exp Ther. 288:421–427. 1999.
Saporito MS., Thomas BA., Scott RW. MPTP activates c-Jun NH(2)-terminal kinase (JNK) and its upstream regulatory kinase MKK4 in nigrostriatal neurons in vivo. J Neurochem. 75:1200–1208. 2000.

Shamoto-Nagai M., Maruyama W., Kato Y., Isobe K., Tanaka M., Naoi M., Osawa T. An inhibitor of mitochondrial complex I, rotenone, inactivates proteasome by oxidative modification and induces aggregation of oxidized proteins in SH-SY5Y cells. J Neurosci Res. 74:589–597. 2003.

Shastry BS. Parkinson disease: etiology, pathogenesis and future of gene therapy. Neurosci Res. 41:5–12. 2001.

Tansey MG., McCoy MK., Frank-Cannon TC. Neuroinflammatory mechanisms in Parkinson's disease: potential environmental triggers, pathways, and targets for early therapeutic intervention. Exp Neurol. 208:1–25. 2007.

Tsai SH., Lin-Shiau SY., Lin JK. Suppression of nitric oxide synthase and the down-regulation of the activation of NfkappaB in macropahages by resveratrol. Br J Pharmacol. 126:673–680. 1999.
Go to : 
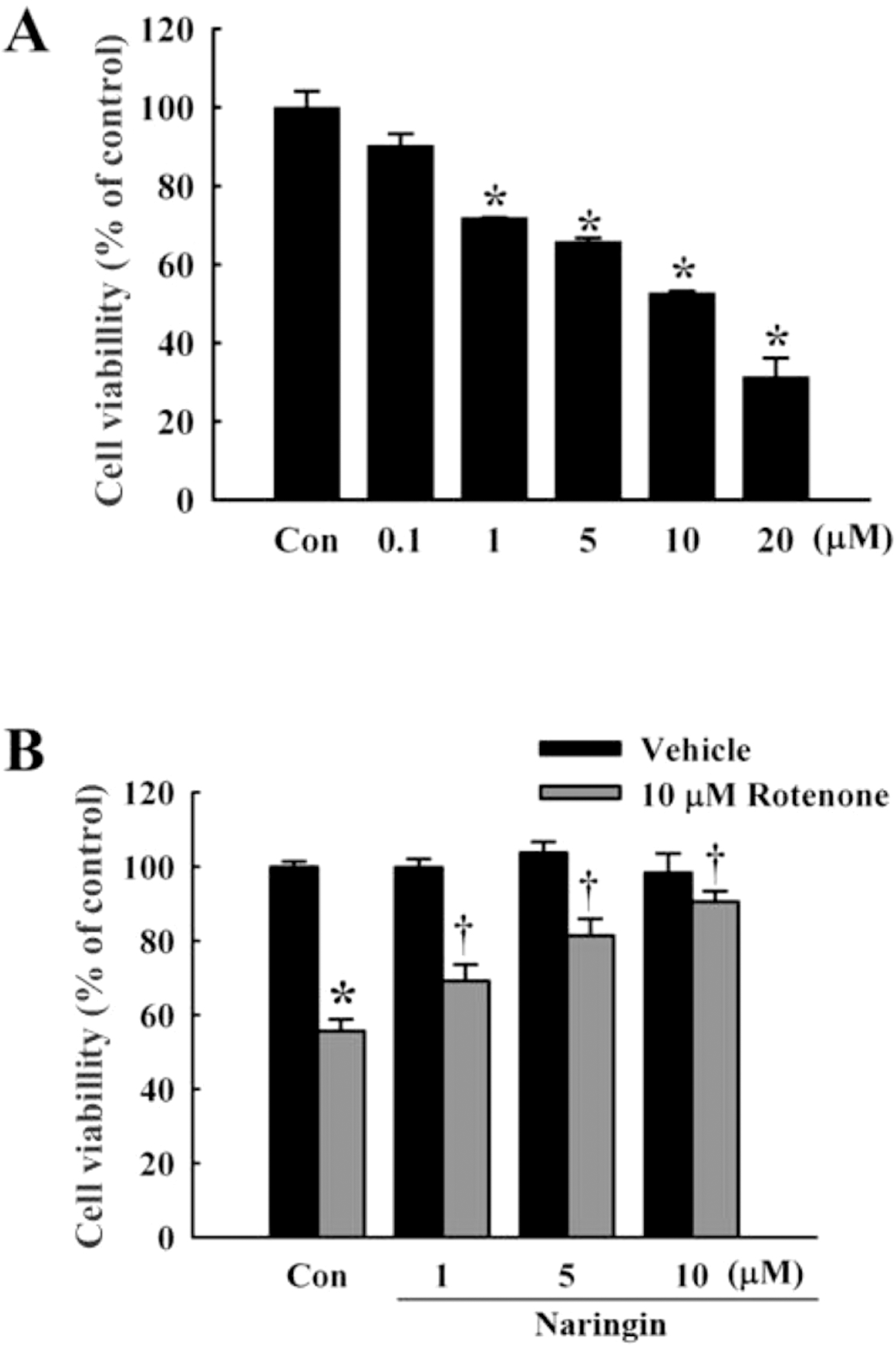 | Fig. 1.Effect of naringin on rotenone-induced cell death in SH-SY5Y cells. (A) MTT cell viability assay at 0.1, 1, 5, 10, and 20 μM rotenone for 24 h in SH-SY5Y cells. (B) Naringin inhibits rotenone-induced cytotoxicity in SH-SY5Y cells, treated with 10 μM rotenone for 24 h. Naringin (1, 5, and 10 μM) was added 4 h before rotenone treatment. Independent experiments were repeated three times. Values are presented as mean±SEM. Con, control. ∗p<0.05 compared to non-treated cells; †p<0.05 compared to rotenone-treated cells. |
 | Fig. 2.Effect of naringin on rotenone-induced apoptotic features in SH-SY5Y cells pretreated with 10 μM rotenone for 24 h. Naringin (10 μM) was treated 4 h prior to rotenone treatment. Condensed chromatin was stained dark brown (lower). Scale bar, 100 μm. Three independent experiments were performed for this study. |
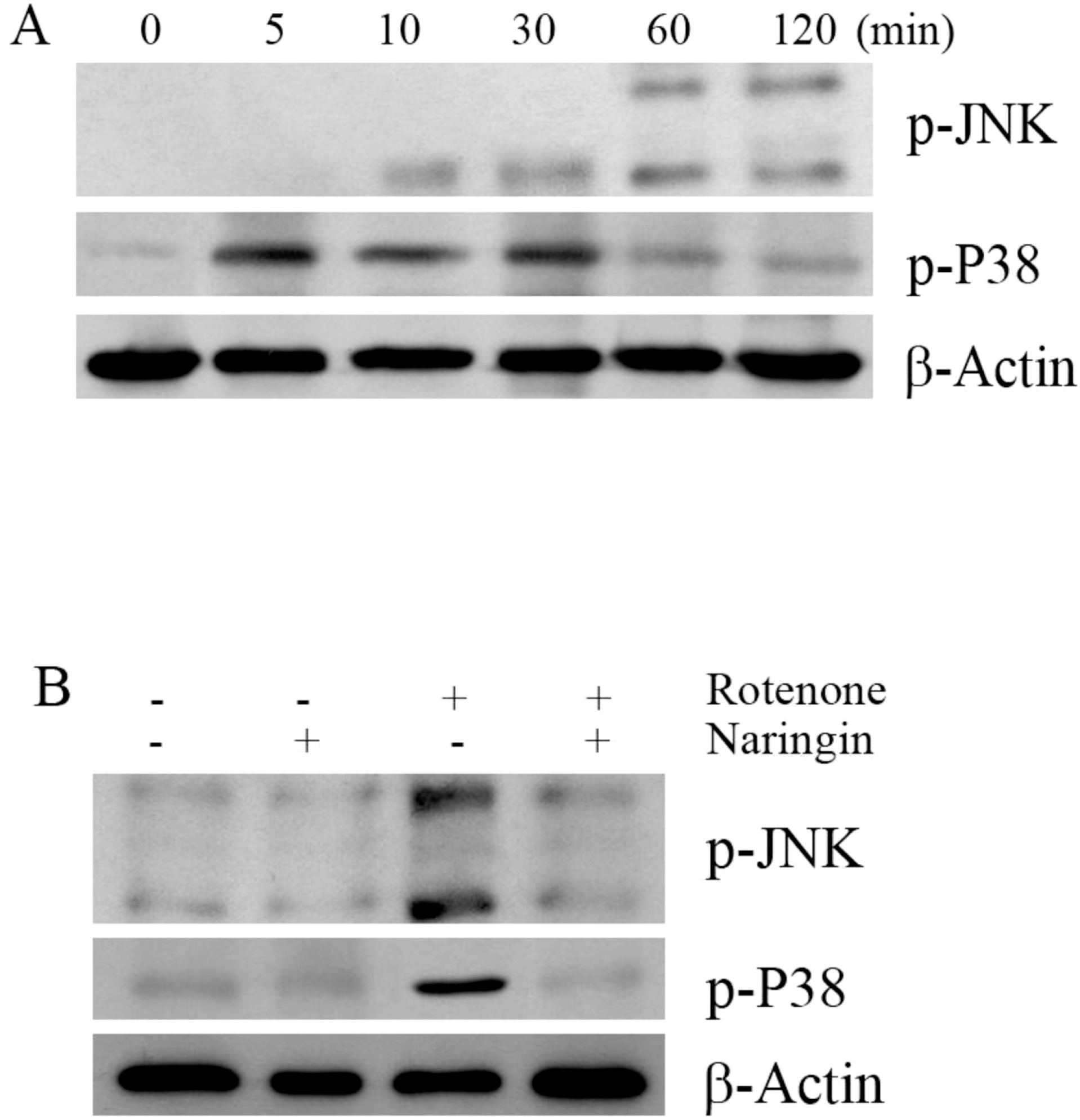 | Fig. 3.The effect of rotenone on the phosphorylation of JNK and P38 in SH-SY5Y cells. (A) The effect of rotenone on the phosphorylation of JNK and P38 by Western blot analysis. Cells were treated with 10 μM rotenone for 120 min. (B) Western blot to determine the effect of naringin on rotenone-induced phosphorylation of JNK and P38 in SH-SY5Y cells. The cells were pretreated with 10 μM rotenone for 120 min, with 10 μM naringin added 4 h prior to rotenone treatment. The cells were harvested for the phosphorylation assay at 30 and 120 min after rotenone treatment. β-Actin was used as an internal standard protein. Three independent experiments were performed for this assay. |
 | Fig. 4.The effect of rotenone on the expression of apoptotic proteins BCL2, BAX, CASP9, PARP, and cleaved CASP3 in SH-SY5Y cells. (A) The effect of rotenone on the expression of BCL2, BAX, CASP9, PARP, and cleaved CASP3 by Western blot analysis. Cells were treated with 10 μM rotenone for 24 h. (B) Western blot assay to determine the effect of naringin on rotenone-induced expression of the apoptotic proteins, BCL2, BAX, cleaved CASP9, cleaved PARP, and cleaved CASP3 in SH-SY5Y cells. Cells were pretreated with 10 μM rotenone for 24 h, with 10 μM naringin added 4 h prior to rotenone treatment. β-Actin was detected as an internal standard protein. (C) Effect of naringin on rotenone-induced activity of CASP3 in SH-SY5Y cells. Cells were pretreated with 100 μM rotenone for 24 h and 10 μM naringin was then added 4 h prior to rotenone treatment. The cleaved CASP3 substrate acetyl-AspGlu-Val-Asp-p-Nitroanilide (Ac-DEVD-p-NA) was measured at 405 nm. CASP3 was used as a positive control. The CASP3 inhibitor (Ac-DEVD-CHO) added with CASP3 was used as negative control. Values are presented as mean±SEM. Three independent experiments were performed for this assay. ∗p<0.05 compared to non-treated cells; †p<0.05 compared to rotenone-treated cells. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download