Abstract
The accumulation of β-amyloid (Aβ) aggregates is a characteristic of Alzheimer's disease (AD). Furthermore, these aggregates have neurotoxic effects on cells, and thus, molecules that inhibit Aβ aggregate formation could be valuable therapeutics for AD. It is well known that aggregation of Aβ depends on its hydrophobicity, and thus, in order to increase the hydrophilicity of Aβ, we considered using citrate, an anionic surfactant with three carboxylic acid groups. We hypothesized that citrate could reduce hydrophobicity and increase hydrophilicity of Aβ1-40 molecules via hydrophilic/electrostatic interactions. We found that citrate significantly inhibited Aβ1-40 aggregation and significantly protected SH-SY5Y cell line against Aβ1-40 aggregates-induced neurotoxicity. In details, we examined the effects of citrate on Aβ1-40 aggregation and on Aβ1-40 aggregates-induced cytotoxicity, cell viability, and apoptosis. Th-T assays showed that citrate significantly inhibited Aβ1-40 aggregation in a concentration-dependent manner (Th-T intensity: from 91.3% in 0.01 mM citrate to 82.1% in 1.0 mM citrate vs. 100.0% in Aβ1-40 alone). In cytotoxicity and viability assays, citrate reduced the toxicity of Aβ1-40 in a concentration-dependent manner, in which the cytotoxicity decreased from 107.5 to 102.3% as compared with Aβ1-40 aggregates alone treated cells (127.3%) and the cell viability increased from 84.6 to 93.8% as compared with the Aβ1-40 aggregates alone treated cells (65.3%). Furthermore, Hoechst 33342 staining showed that citrate (1.0 mM) suppressed Aβ1-40 aggregates-induced apoptosis in the cells. This study suggests that citrate can inhibit Aβ1-40 aggregation and protect neurons from the apoptotic effects of Aβ1-40 aggregates. Accordingly, our findings suggest that citrate administration should be viewed as a novel neuroprotective strategy for AD.
REFERENCES
Ban T., Hoshino M., Takahashi S., Hamada D., Hasegawa K., Naiki H., Goto Y. Direct observation of Abeta amyloid fibril growth and inhibition. J Mol Biol. 344:757–767. 2004.
Barrow CJ., Zagorski MG. Solution structures of beta peptide and its constituent fragments: relation to amyloid deposition. Science. 253:179–182. 1991.
Bokvist M., Lindström F., Watts A., Gröbner G. Two types of Alzheimer's beta-amyloid (1-40) peptide membrane interactions: aggregation preventing transmembrane anchoring versus accelerated surface fibril formation. J Mol Biol. 335:1039–1049. 2004.
Brus L. Noble metal nanocrystals: plasmon electron transfer photochemistry and single-molecule raman spectroscopy. Acc Chem Res. 41:1742–1749. 2008.

Bush AI., Pettingell WH., Multhaup G., d Paradis M., Vonsattel JP., Gusella JF., Beyreuther K., Masters CL., Tanzi RE. Rapid induction of Alzheimer Abeta amyloid formation by zinc. Science. 265:1464–1467. 1994.
Cao M., Han Y., Wang J., Wang Y. Modulation of fibrillogenesis of amyloid β (1-40) peptide with cationic gemini surfactant. J Phys Chem B. 111:13436–13443. 2007.
Citron M. Strategies for disease modification in Alzheimer's disease. Nat Rev Neurosci. 5:677–685. 2004.

Cummings JL., Doody R., Clark C. Disease-modifying therapies for Alzheimer disease: challenges to early intervention. Neurology. 69:1622–1634. 2007.

Gschwind M., Huber G. Apoptotic cell death induced by beta-amyloid 1-42 peptide is cell type dependent. J Neurochem. 65:292–300. 1995.
Halverson K., Fraser PE., Kirschner DA., Lansbury PT Jr. Molecular determinants of amyloid deposition in Alzheimer's disease: conformational studies of synthetic beta-protein fragments. Biochemistry. 29:2639–2644. 1990.

Hensley K., Carney JM., Mattson MP., Aksenova MV., Harris ME., Wu JF., Floyd RA., Butterfield DA. A model for β-amyloid aggregation and neurotoxicity based on free radical generation by the peptide: relevance to Alzheimer disease. Proc Natl Acad Sci USA. 91:3270–3274. 1994.
Howlett DR., Jennings KH., Lee DC., Clark MS., Brown F., Wetzel R., Wood SJ., Camilleri P., Roberts GW. Aggregation state and neurotoxic properties of Alzheimer beta-amyloid peptide. Neurodegeneration. 4:23–32. 1995.

Jarrett JT., Berger EP., Lansbury Jr. PT. The carboxy terminus of the β-amyloid protein is critical for the seeding of amyloid formation: implication for the pathogenesis of Alzheimer's disease. Biochemistry. 32:4693–4697. 1993.
Jeng US., Lin TL., Lin JM., Ho DL. Contrast variation SANS for the solution structure of the β-amyloid peptide1-40 influenced by SDS surfactants. Physica B. 385-386:865–867. 2006.
Ji SR., Wu Y., Sui SF. Study of beta-amyloid peptide (Abeta40) insertion into phospholipid membranes using monolayer technique. Biochemistry (Mosc). 67:1283–1288. 2002.
Khandogin J., Brooks III CL. Linking folding with aggregation in Alzheimer's beta-amyloid peptides. Proc Natl Acad Sci USA. 104:16880–16885. 2007.

Kisilevsky R., Lemieux LJ., Fraser PE., Kong X., Hultin PG., Szarek WA. Arresting amyloidosis in vivo using small-molecule anionic sulphonates or sulphates: implications for Alzheimer's disease. Nat Med. 1:143–148. 1995.

Koh JY., Yang LLY., Cotman CW. Beta-amyloid protein increases vulnerability of cultured neurons to excitotoxins. Brain Res. 533:315–320. 1990.
Lazo ND., Grant MA., Condron MC., Rigby AC., Teplow DB. On the nucleation of amyloid beta-protein monomer folding. Protein Sci. 14:1581–1596. 2005.
Lizard G., Fournel S., Genestier L., Dhedin N., Chaput C., Flacher M., Mutin M., Panaye G., Revillard JP. Kinetics of plasma membrane and mitochondrial alterations in cells undergoing apoptosis. Cytometry. 21:275–283. 1995.

Lomakin A., Chung DS., Benedek GB., Kirschner DA., Teplow DB. On the nucleation and growth of amyloid beta-protein fibrils: Detection of nuclei and quantitation of rate constants. Proc Natl Acad Sci USA. 93:1125–1129. 1996.

Lorenzo A., Yankner BA. Beta-amyloid neurotoxicity requires fibril formation and is inhibited by Congo red. Proc Natl Acad Sci USA. 91:12243–12247. 1994.

Lowe TL., Strzelec A., Kiessling LL., Murphy RM. Structure-function relationships for inhibitors of beta-amyloid toxicity containing the recognition sequence KLVFF. Biochemistry. 40:7882–7889. 2001.
Lührs T., Ritter C., Adrian M., Riek- Loher D., Bohrmann B., Dobeli H., Schubert D., Riek R. 3D structure of Alzheimer's amyloid-β (1-42) fibrils. Proc Natl Acad Sci USA. 102:17342–17347. 2005.
Marcinowski KJ., Shao H., Clancy EL., Zagorski MG. Solution structure model of residues 1-28 of the amyloid beta peptide when bound to micelles. J Am Chem Soc. 120:11082–11091. 1998.
McConnell S., Riggs J. Alzheimer's research: can it help save our nation's entitlement programs? Alzheimer Dementia. 1:84–86. 2005.

Mook-Jung I., Joo I., Sohn S., Kwon HJ., Huh K., Jung MW. Estrogen blocks neurotoxic effects of beta-amyloid (1-42) and induces neurite extension in B103 cells. Neurosci Lett. 235:101–104. 1997.
Nowick JS., Lam KS., Khasanova TV., Kemnitzer WE., Maitra S., Mee HT., Liu R. An unnatural amino acid that induces beta-sheet folding and interaction in peptides. J Am Chem Soc. 124:4972–4973. 2002.
Pallitto MM., Ghanta J., Heinzelman P., Kiessling LL., Murphy RM. Recognition sequence design for peptidyl modulators of beta-amyloid aggregation and toxicity. Biochemistry. 38:3570–3578. 1999.
Pappolla M., Bozner P., Soto C., Shao H., Robakis NK., Zagorski M., Frangione B., Ghiso J. Inhibition of Alzheimer beta-fibrillo-genesis by melatonin. J Biol Chem. 273:7185–7188. 1998.
Pereira C., Agostinho P., Moreira PI., Cardoso SM., Oliveira CR. Alzheimer's disease-associated neurotoxic mechanisms and neuroprotective strategies. Curr Drug Targets CNS Neurol Disord. 4:383–403. 2005.

Pike CJ., burdick D., Walencewicz AJ., Glabe CG., Cotman CW. Neurodegeneration induced by β-amyloid peptides in vitro: the role of peptide assembly state. J Neruosci. 13:1676–1687. 1993.
Pike CJ., Walencewicz AJ., Glabe CG., Cotman CW. Aggregation related toxicity of synthetic beta-amyloid protein in hippocampal cultures. Eur J Pharmacol. 207:367–368. 1991.
Pike CJ., Walencewicz-Wasserman AJ., Kosmoski J., Cribbs DH., Glabe CG., Cotman CW. Structure-activity analyses of beta-amyloid peptides: contributions of the beta 25-35 region to aggregation and neurotoxicity. J Neurochem. 64:253–265. 1995.
Puntel RL., Roos DH., Grotto D., Garcia SC., Nogueira CW., Rocha JB. Antioxidant properties of Krebs cycle intermediates against malonate pro-oxidant activity in vitro: a comparative study using the colorimetric method and HPLC analysis to determine malondialdehyde in rat brain homogenates. Life Sci. 81:51–62. 2007.

Rangachari V., Reed DK., Moore BD., Rosenberry TL. Secondary structure and interfacial aggregation of amyloid-beta (1-40) on sodium dodecyl sulfate micelles. Biochemistry. 45:8639–8648. 2006.
Rochet JC Jr., Lansbury PT. Amyloid fibrillogenesis: themes and variations. Curr Opin Struct Biol. 10:60–68. 2000.

Sabate R., Gallardo M., Estelrich J. An autocatalytic reaction as a model for the kinetics of the aggregation of beta-amyloid. Biopolymers. 71:190–195. 2003.
Sabaté R., Estelrich J. Evidence of the existence of micelles in the fibrillogenesis of beta-amyloid peptide. J Phys Chem B. 109:11027–11032. 2005a.
Sabaté R., Estelrich J. Stimulatory and inhibitory effects of alkyl bromide surfactants on beta-amyloid fibrillogenesis. Langmuir. 21:6944–6949. 2005b.
Salomon AR., Marcinowski KJ., Friedland RW., Zagorski MG. Nicotine inhibits amyloid formation by the beta-peptide. Biochemistry. 35:13568–13578. 1996.
Scarpini E., Scheltens P., Feldman H. Treatment of Alzheimer's disease: current status and new perspectives. Lancet Neurol. 2:539–547. 2003.

Seiffert D., Bradley JD., Rominger CM., Rominger DH., Yang F., Meredith JE Jr., Wang Q., Roach AH., Thompson LA., Spitz SM., Higaki JN., Prakash SR., Combs AP., Copeland RA., Arneric SP., Hartig PR., Robertson DW., Cordell B., Stern AM., Olson RE., Zaczek R. Presenilin-1 and −2 are molecular targets for gamma-secretase inhibitors. J Biol Chem. 275:34086–34091. 2000.
Seilheimer B., Bohrmann B., Bondolfi L., Müller F., Stüber D., Döbeli H. The toxicity of the Alzheimer's beta-amyloid peptide correlates with a distinct fiber morphology. J Struct Biol. 119:59–71. 1997.
Sisodia SS., St George-Hyslop PH. Gamma-Secretase, Notch, Abeta and Alzheimer's disease: where do the presenilins fit in? Nat Rev Neurosci. 3:281–290. 2002.
Snyder SW., Ladror US., Wade WS., Wang GT., Barrett LW., Matayoshi ED., Huffaker HJ., Krafft GA., Holzman TF. Amyloidbeta aggregation: selective inhibition of aggregation in mixtures of amyloid with different chain lengths. Biophys J. 67:1216–1228. 1994.

Soreghan B., Kosmoski J., Glabe C. Surfactant properties of Alzheimer's Abeta peptides and the mechanism of amyloid aggregation. J Biol Chem. 269:28551–28554. 1994.
Wang SS., Chen YT., Chou SW. Inhibition of amyloid fibril formation of beta-amyloid peptides via the amphiphilic surfactants. Biochim Biophys Acta. 1741:307–313. 2005.
Watanabe K., Segawa T., Nakamura K., Kodaka M., Konakahara T., Okuno H. Identification of the molecular-interaction site of amyloid beta peptide by using a fluorescence assay. J Pept Res. 58:342–346. 2001.
Wood SJ., MacKenzie L., Maleeff B., Hurle MR., Wetzel R. Selective inhibition of Abeta fibril formation. J Biol Chem. 271:4086–4092. 1996a.
Wood SJ., Maleeff B., Hart T., Wetzel R. Physical, morphological and functional differences between ph 5.8 and 7.4 aggregates of the Alzheimer's amyloid peptide Abeta. J Mol Biol. 256:870–877. 1996b.
Yang HD., Son IH., Lee SS., Park YH. Protective effect of citrate against Aβ-induced neurotoxicity in PC12 cells. Mol Cell Toxicol. 4:157–163. 2008.
Fig. 1.
The effect of citrate on aggregate formation of Aβ1-40 was determined by Th-T fluorometric assays. Aβ1-40 at 20 μM was incubated with citrate at 0.01, 0.1, or 1.0 mM at 37°C for 6 days. The fluorescence intensities of Aβ1-40 aggregate formation were measured using a spectrofluorometer. Results are means±SDs of three experiments. The asterisk (∗) indicates a significant (p<0.05) difference between treatments with Aβ aggregates alone and Aβ plus citrate.
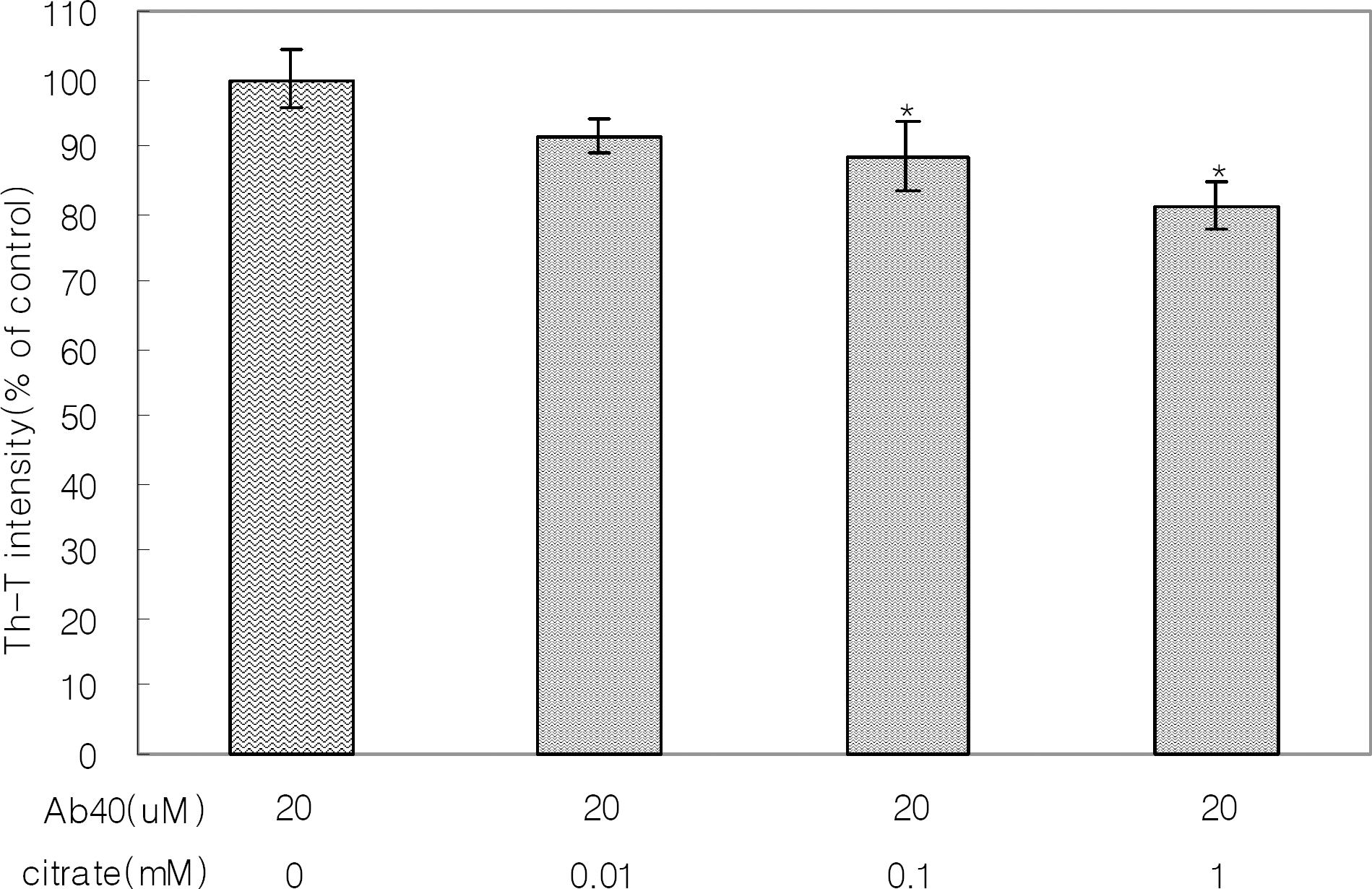
Fig. 2.
The effect of citrate on the viability of SH-SY5Y cell line. The cells were treated for 3 days with the solution of 20 μM Aβ1-40 with/without citrate preincubated at 37°C for 6 days. Results are means±SDs of three experiments. The asterisk (∗) indicates a significant (p<0.05) difference between treatment with Aβ alone and treatment with Aβ plus citrate.
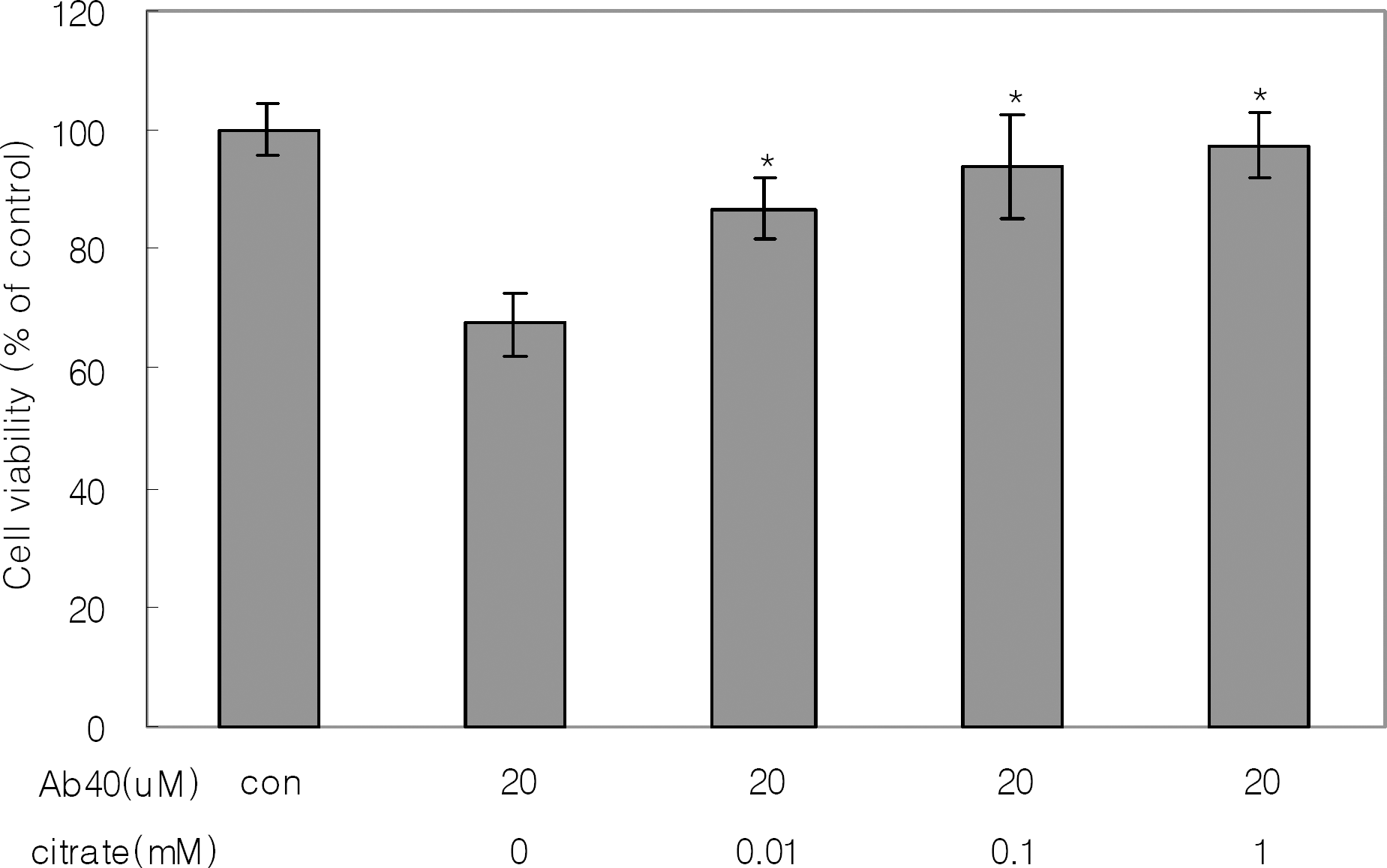
Fig. 3.
The protective effect of citrate on Aβ1-40 aggregates-induced neuronal damage of SH-SY5Y cell line. The cells were treated for 3 days with the solution of 20 μM Aβ1-40 with/without citrate preincubated at 37°C for 6 days. Results are means±SDs of three experiments. The asterisk (∗) indicates a significant (p<0.05) difference between treatment with Aβ alone and treatment with Aβ plus citrate.
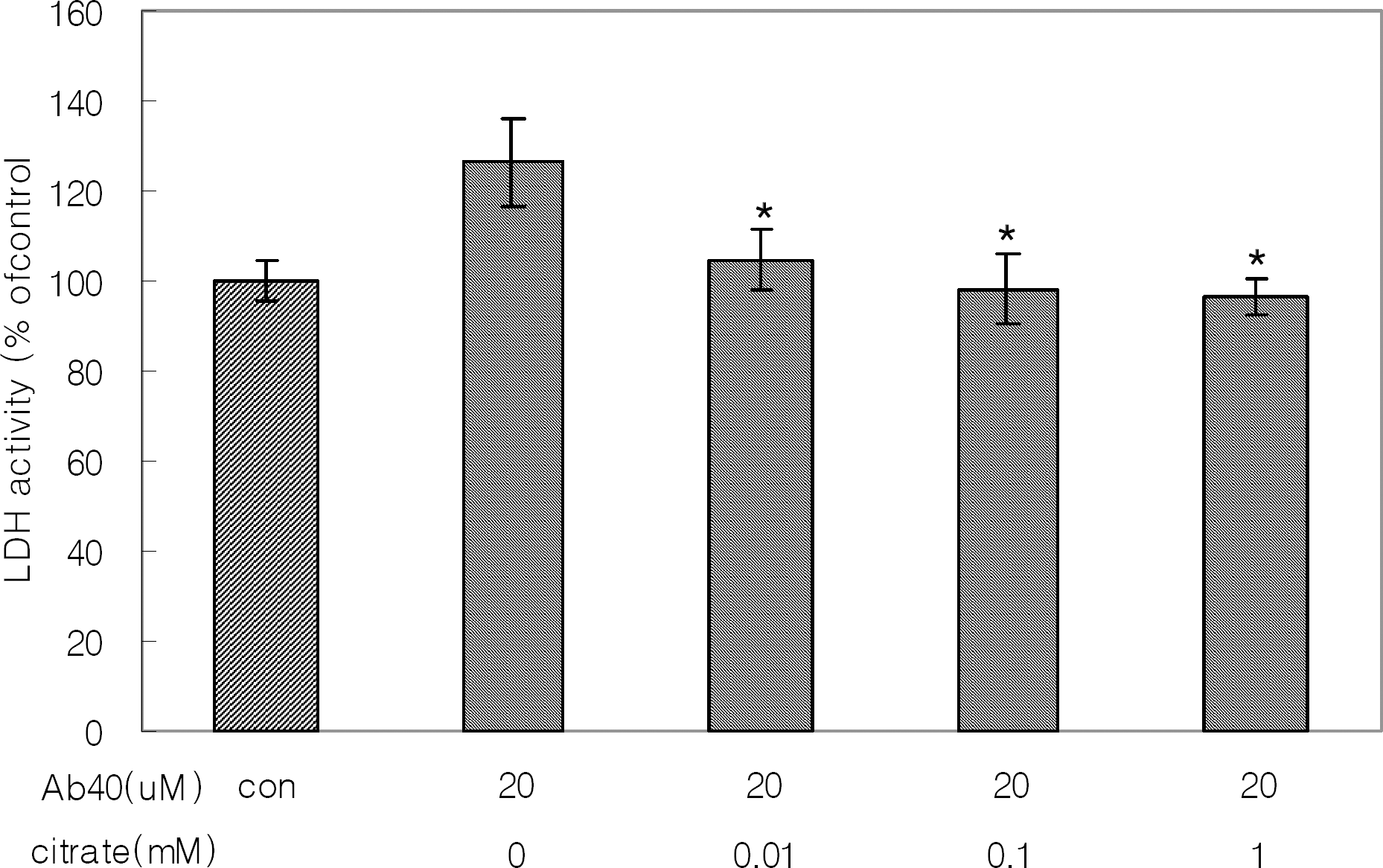
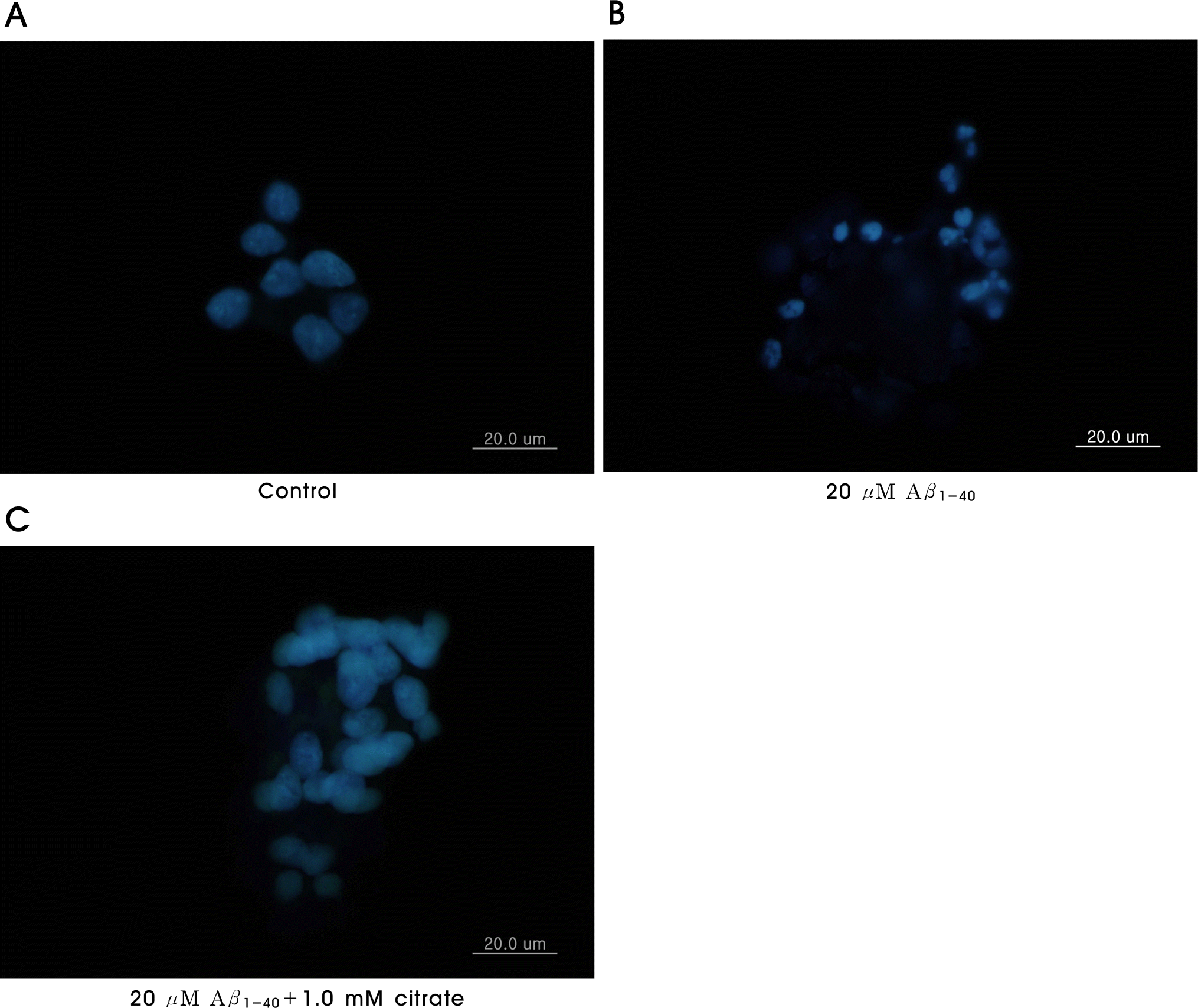
Fig. 4.
Inhibitory effect of citrate on Aβ1-40-induced apoptosis. Apoptotic body formation was observed under a fluorescent microscope after Hoechst 33342 staining. Hoechst labeling shows (A) control (untreated cells), (B) increased nuclear condensation and fragmentation in 20 μM Aβ1-40 treated cells and (C) decreased nuclear condensation and fragmentation in cells treated with 20 μM Aβ1-40 plus 1.0 mM citrate preincubated for 6 days. The cells were labeled with 10 μg/ml Hoechst 33342 for 1 h after exposure to Aβ1-40 treatments for 3 days. Original magnification: ×1,000.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download