Abstract
Nitric oxide (NO) has both neuroprotective and neurotoxic effects depending on its concentration and the experimental model. We tested the effects of NG-nitro-L-arginine methyl ester (L-NAME), a nonselective nitric oxide synthase (NOS) inhibitor, and aminoguanidine, a selective inducible NOS (iNOS) inhibitor, on kainic acid (KA)-induced seizures and hippocampal CA3 neuronal death. L-NAME (50 mg/kg, i.p.) and/or aminoguanidine (200 mg/kg, i.p.) were administered 1 h prior to the intracerebroventricular (i.c.v.) injection of KA. Pretreatment with L-NAME significantly increased KA-induced CA3 neuronal death, iNOS expression, and activation of microglia. However, pretreatment with aminoguanidine significantly suppressed both the KA-induced and L-NAME-aggravated hippocampal CA3 neuronal death with concomitant decreases in iNOS expression and microglial activation. The protective effect of aminoguanidine was maintained for up to 2 weeks. Furthermore, iNOS knockout mice (iNOS−/–) were resistant to KA-induced neuronal death. The present study demonstrates that aminoguanidine attenuates KA-induced neuronal death, whereas L-NAME aggravates neuronal death, in the CA3 region of the hippocampus, suggesting that NOS isoforms play different roles in KA-induced excitotoxicity.
Go to : 
REFERENCES
Alderton WK., Cooper CE., Knowles RG. Nitric oxide synthases: structure, function and inhibition. Biochem J. 357:593–615. 2001.

Baker H., Farbman AI. Olfactory afferent regulation of the dopamine phenotype in the fetal rat olfactory system. Neuroscience. 52:115–134. 1993.

Boer R UMPW., Klein T., Mirau B., Haas S., Baur I. The inhibitory potency and selectivity of arginine substrate site nitric-oxide synthase inhibitors is solely determined by their affinity toward the different isoenzymes. Mol Pharmacol. 58:1026–1034. 2000.

Buisson A., Lakhmeche N., Verrecchia C., Plotkine M., Boulu RG. Nitric oxide: an endogenous anticonvulsant substance. Neuroreport. 4:444–446. 1993a.
Buisson A., Margaill I., Callebert J., Plotkine M., Boulu RG. Mechanisms involved in the neuroprotective activity of a nitric oxide synthase inhibitor during focal cerebral ischemia. J Neurochem. 61:690–696. 1993b.

Ciani E., Baldinotti I., Contestabile A. Sustained, long-lasting inhibition of nitric oxide synthase aggravates the neural damage in some models of excitotoxic brain injury. Brain Res Bull. 56:29–35. 2001.

Coyle JT., Puttfarcken P. Oxidative stress, glutamate, and neurodegenerative disorders. Science. 262:689–695. 1993.

Danielisova V., Nemethova M., Burda J. The protective effect of aminoguanidine on cerebral ischemic damage in the rat brain. Physiol Res. 53:533–540. 2004.
Du W., Harvey JA. The nitric oxide synthesis inhibitor L-NAME facilitates associative learning. Prog Neuropsychopharmacol Biol Psychiatry. 20:1183–1195. 1996.

Du W., Weiss H., Harvey JA. Associative learning is enhanced by selective neuronal nitric oxide synthase inhibitors and retarded by a nitric oxide donor in the rabbit. Psychopharmacology (Berl). 150:264–271. 2000.

Garthwaite J., Boulton CL. Nitric oxide signaling in the central nervous system. Annu Rev Physiol. 57:683–706. 1995.

Gross SS., Wolin MS. Nitric oxide: pathophysiological mechanisms. Annu Rev Physiol. 57:737–769. 1995.

Haberny KA., Pou S., Eccles CU. Potentiation of quinolinate-induced hippocampal lesions by inhibition of NO synthesis. Neurosci Lett. 146:187–190. 1992.

Henshall DC., Bonislawski DP., Skradski SL., Araki T., Lan JQ., Schindler CK., Meller R., Simon RP. Formation of the Apaf-1/cytochrome c complex precedes activation of caspase-9 during seizure-induced neuronal death. Cell Death Differ. 8:1169–1181. 2001.

Jones PA., Smith RA., Stone TW. Nitric oxide synthase inhibitors L-NAME and 7-nitroindazole protect rat hippocampus against kainate-induced excitotoxicity. Neurosci Lett. 249:75–78. 1998.

Kiedrowski L., Costa E., Wroblewski JT. Glutamate receptor agonists stimulate nitric oxide synthase in primary cultures of cerebellar granule cells. J Neurochem. 58:335–341. 1992.

Kim YM., Bombeck CA., Billiar TR. Nitric oxide as a bifunctional regulator of apoptosis. Circ Res. 84:253–256. 1999.

Koprowski H., Zheng YM., Heber-Katz E., Fraser N., Rorke L., Fu ZF., Hanlon C., Dietzschold B. In vivo expression of inducible nitric oxide synthase in experimentally induced neurologic diseases. Proc Natl Acad Sci USA. 90:3024–3027. 1993.

Laursen SE., Belknap JK. Intracerebroventricular injections in mice. Some methodological refinements. J Pharmacol Methods. 16:355–357. 1986.
Lee JM., Zipfel GJ., Choi DW. The changing landscape of ischaemic brain injury mechanisms. Nature. 399:A7–14. 1999.

Moncada S., Higgs A., Furchgott R. International Union of Pharmacology Nomenclature in Nitric Oxide Research. Pharmacol Rev. 49:137–142. 1997.
Mulsch A., Busse R., Mordvintcev PI., Vanin AF., Nielsen EO., Scheel-Kruger J., Olesen SP. Nitric oxide promotes seizure activity in kainate-treated rats. Neuroreport. 5:2325–2328. 1994.

Penix LP., Davis W., Subramaniam S. Inhibition of NO synthase increases the severity of kainic acid-induced seizures in rodents. Epilepsy Res. 18:177–184. 1994.

Perez-Asensio FJ., Hurtado O., Burguete MC., Moro MA., Salom JB., Lizasoain I., Torregrosa G., Leza JC., Alborch E., Castillo J., Knowles RG., Lorenzo P. Inhibition of iNOS activity by 1400W decreases glutamate release and ameliorates stroke outcome after experimental ischemia. Neurobiol Dis. 18:375–384. 2005.
Rondouin G., Bockaert J., Lerner-Natoli M. L-nitroarginine, an inhibitor of NO synthase, dramatically worsens limbic epilepsy in rats. Neuroreport. 4:1187–1190. 1993.
Schulz JB., Matthews RT., Jenkins BG., Ferrante RJ., Siwek D., Henshaw DR., Cipolloni PB., Mecocci P., Kowall NW., Rosen BR. Blockade of neuronal nitric oxide synthase protects against excitotoxicity. in vivo. J Neurosci. 15:8419–8429. 1995.
Shapira S., Kadar T., Weissman BA. Dose-dependent effect of nitric oxide synthase inhibition following transient forebrain ischemia in gerbils. Brain Res. 668:80–84. 1994.

Southam E., Garthwaite J. The nitric oxide-cyclic GMP signalling pathway in rat brain. Neuropharmacology. 32:1267–1277. 1993.

Starr MS., Starr BS. Paradoxical facilitation of pilocarpine-induced seizures in the mouse by MK-801 and the nitric oxide synthesis inhibitor L-NAME. Pharmacol Biochem Behav. 45:321–325. 1993.

Sugimoto K., Iadecola C. Effects of aminoguanidine on cerebral ischemia in mice: comparison between mice with and without inducible nitric oxide synthase gene. Neurosci Lett. 331:25–28. 2002.

Takei Y., Takashima S., Ohyu J., Matsuura K., Katoh N., Takami T., Miyajima T., Hoshika A. Different effects between 7-nitroindazole and L-NAME on cerebral hemodynamics and hippocampal lesions during kainic acid-induced seizures in newborn rabbits. Brain Dev. 23:406–413. 2001.

Takei Y., Takashima S., Ohyu J., Takami T., Miyajima T., Hoshika A. Effects of nitric oxide synthase inhibition on the cerebral circulation and brain damage during kainic acid-induced seizures in newborn rabbits. Brain Dev. 21:253–259. 1999.

Go to : 
 | Fig. 1.Effects of NOS inhibitors on KA-induced neuronal cell death in the CA3 region of mouse hippocampus. Neuronal cell death was determined with cresyl violet staining. KA injection produced a marked loss of pyramidal neurons (IA, E) and L-NAME treatment prior to KA (IB, F) potentiated this KA-induced neuronal loss. Aminoguanidine pretreatment (IC, G) markedly attenuated KA-induced and L-NAME-aggravated (ID, H) neuronal death. Neuronal cell death was not observed in vehicle-, aminoguanidine-only-, or L-NAME-only-treated mice (II). Images in the upper right corner show the entire hippocampus and boxes in these images indicate the enlarged views of the CA3 region. Arrows indicate areas where most neuronal death appears. KA & K stand for kainic acid, L for L-NAME, and A for aminoguanidine. Scale bar: 100 μm. |
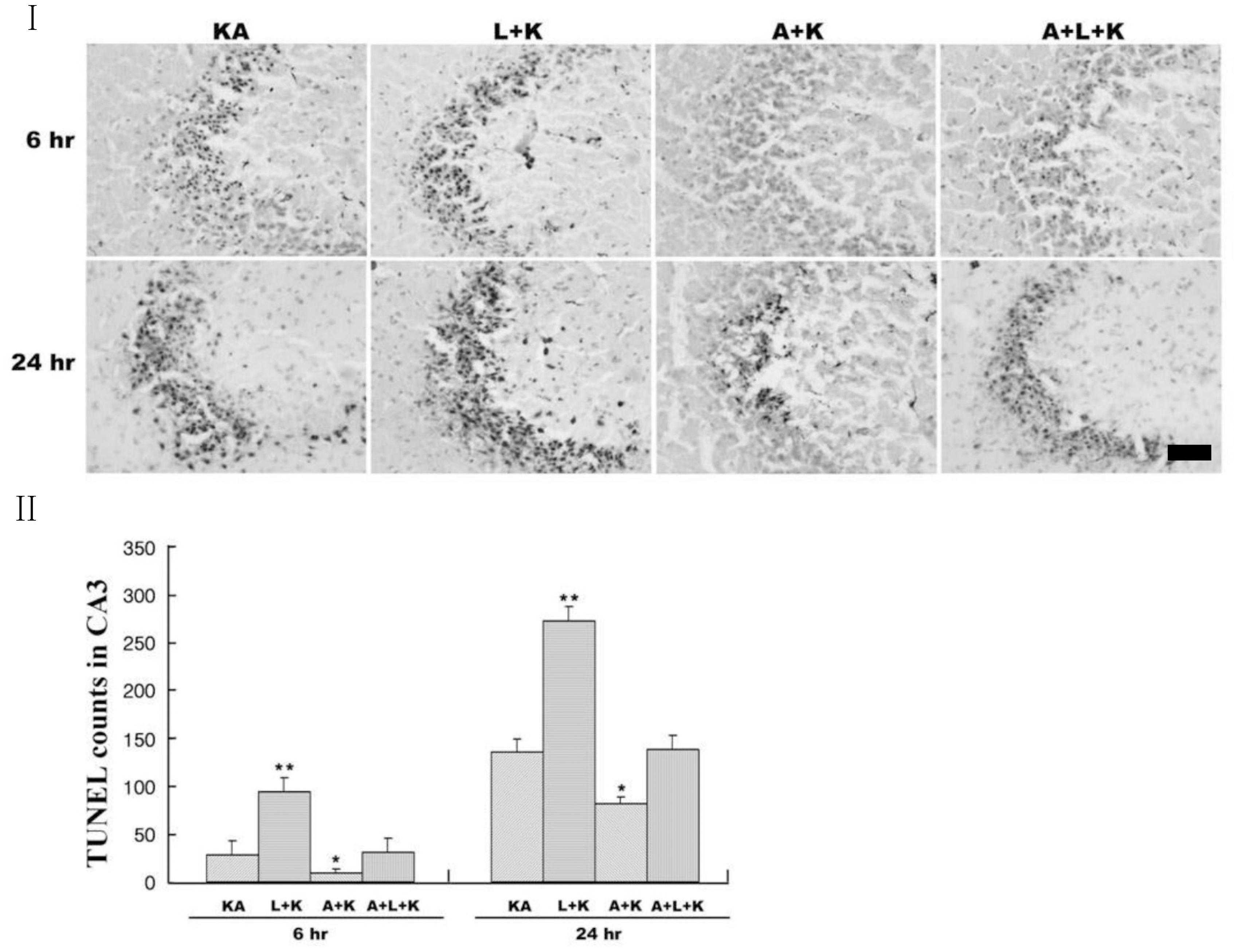 | Fig. 2.Representative (I) and quantitative (II) neuronal death measured with Terminal deoxytransferase-mediated dUTP-nick end labeling (TUNEL) assay. KA produces TUNEL-positive neurons within 24 h, and L-NAME (L+KA) treatment potentiates this induction. Aminoguanidine (A+KA) reduces the numbers of TUNEL-positive cells compared with KA-only (KA) and L-NAME-aggravated (A+L+KA) groups. L-NAME and aminoguanidine alone did not affect cell survival (data not shown). Quantitative data represent three independent experiments and are expressed as mean±SEM. ∗p<0.05, ∗∗p<0.01 versus the KA-only treated group. KA & K stand for kainic acid, L for L-NAME, and A for aminoguanidine. |
 | Fig. 3.Aminoguanidine produces persistent neuronal survival against KA and L-NAME injury. Pyramidal neurons were measured with cresyl violet staining (Top panels) and NeuN immunoreactivity (Bottom panels) at 2 weeks after KA injection. Arrows indicate areas with highest neuronal death. KA & K stand for kainic acid, L for L-NAME, A for aminoguanidine. |
 | Fig. 4.Effects of NOS inhibitors on KA-induced microglial activation and subsequent iNOS expression. Expression of iNOS (top panels) and OX-6, a microglial marker, (bottom panels) within the CA3 was determined with immunohistochemical analysis at 24 h after treatment. KA increased iNOS expression (A) and microglial activation (E), and these phenomena were attenuated with aminoguanidine pretreatment (C, G). L-NAME pretreatment (B, F) potentiated the KA effect, but aminoguanidine reduced this potentiation (D, H). L-NAME and aminoguanidine alone did not affect microglial activation and iNOS expression (data not shown). KA & K stand for kainic acid, L for L-NAME, and A for aminoguanidine. |
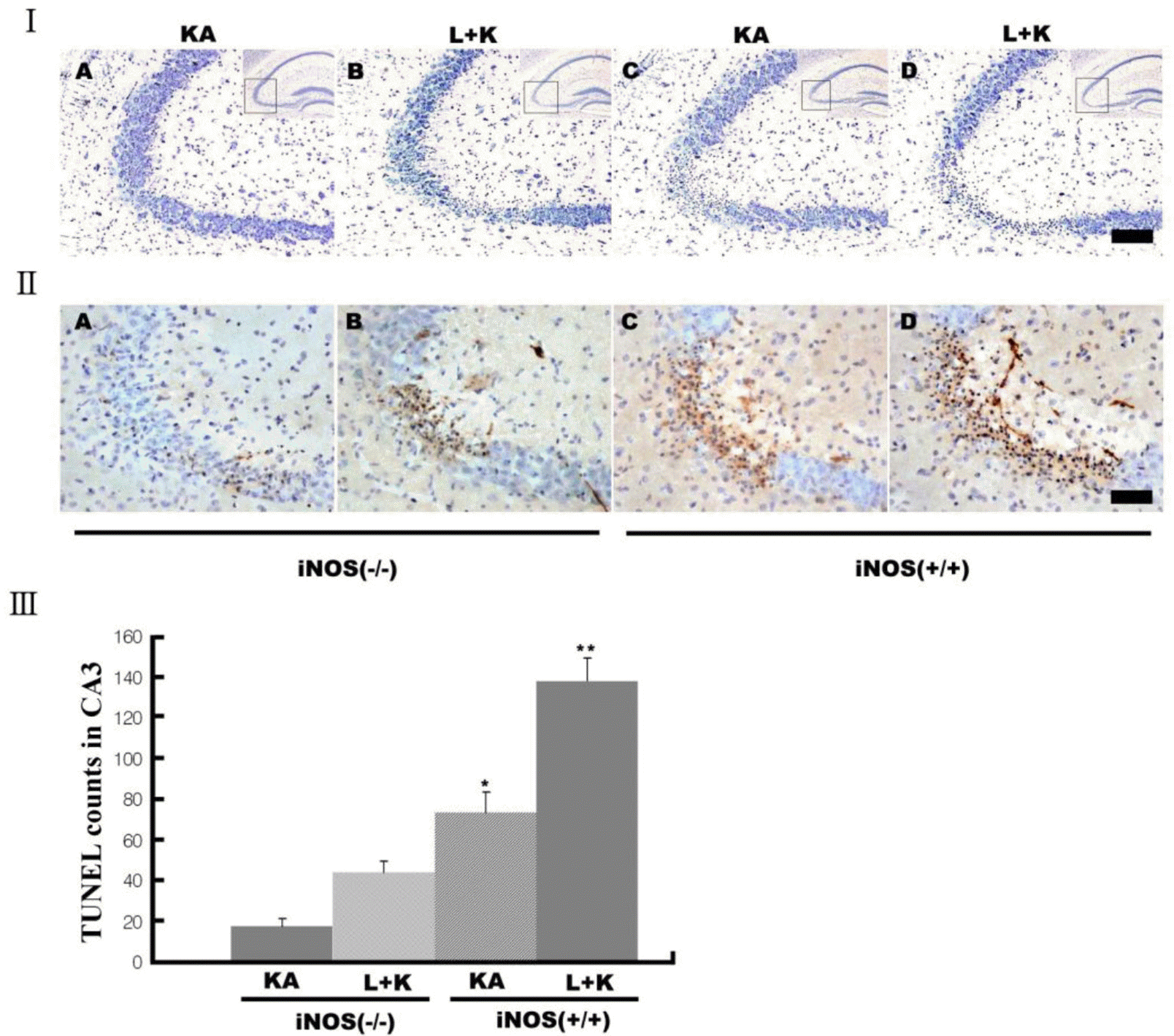 | Fig. 5.Decreased neuronal death after KA in iNOS knockout (iNOS−/–) mice. Neuronal death was measured with cresy violet (I) and TUNEL staining (II&III). KA produced less neuronal death in iNOS−/– mice (I&II-As) than littermates (I&II-Cs). L-NAME pretreatment potentiated neuronal death in both littermates (I&II-Ds) and iNOS−/– mice (I&II-Bs) compared to KA alone, but iNOS−/– mice showed less potentiation (III). Quantitative data represent three independent experiments and are expressed as mean±SEM. ∗∗p<0.01 versus KA-only treated iNOS−/– mice. Images in the upper right corner show the entire hippocampus and boxes in these images indicate the enlarged views of the CA3 region. KA & K stand for kainic acid, L for L-NAME, and A for aminoguanidine. Scale bars: I, 100 μm; II, 50 μm. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download