Abstract
This study aimed to investigate whether selective serotonin reuptake inhibitors (SSRIs) attenuate brain injury and facilitate recovery following photothrombotic cortical ischemia in mice. Male ICR mice were anesthetized and systemically administered Rose Bengal. Permanent focal ischemia was induced in the medial frontal and somatosensory cortices by irradiating the skull with cold light laser. The animals were treated with fluoxetine or sertraline once a day for 14 d starting 1 h after ischemic insult. Treatment with fluoxetine and sertraline significantly reduced the infarct size. The Evans blue extravasation indices of the fluoxetine- and sertraline-treated groups were significantly lower than that of the vehicle group. Treatment with fluoxetine and sertraline shifted the lower limit of the mean arterial blood pressure for cerebral blood flow autoregulation toward normal, and significantly increased the expression of heme oxygenase-1 (HO-1) and hypoxia-inducible factor-1α (HIF-1α) proteins in the ischemic region. These results suggest that SSRIs, such as fluoxetine and sertraline, facilitate recovery following photothrombotic cortical ischemia via enhancement of HO-1 and HIF-1α proteins expression, thereby providing a benefit in therapy of cerebral ischemia.
Go to : 
REFERENCES
Acker T., Acker H. Cellular oxygen sensing need in CNS function: physiological and pathological implications. J Exp Biol. 207:3171–3188. 2004.

Andersen G., Vestergaard K., Riis J., Lauritzen L. Incidence of post-stroke depression during the first year in a large unselected stroke population determined using a valid standardized rating scale. Acta Psychiatr Scand. 90:190–195. 1994b.

Arboix A., Garcia-Eroles L., Comes E., Oliveres M., Balcells M., Pacheco G., Targa C. Predicting spontaneous early neurological recovery after acute ischemic stroke. Eur J Neurol. 10:429–435. 2003.

Bederson JB., Pitts LH., Germano SM., Nishimura MC., Davis RL., Bartkowski HM. Evaluation of 2,3,5-triphenyltetrazolium chloride as a stain for detection and quantification of experimental cerebral infarction in rats. Stroke. 17:1304–1308. 1986.

Betz AL. Vasogenic brain edema. Welch KMA, Caplan LR, Reis DJ, Siesj BK, Weir B, editors. ed,. Primer on cerebrovascular diseases. San Diego: Academic Press;p. p. 56–159. 1997.
Bhardwaj A., Alkayed NJ., Kirsch JR., Hurn PD. Mechanisms of ischemic brain damage. Curr Cardiol Rep. 5:160–167. 2003.

Bhogal SK., Teasell R., Foley N., Speechley M. Heterocyclics and selective serotonin reuptake inhibitors in the treatment and prevention of post-stroke depression. J Am Geriatr Soc. 53:1051–1057. 2005.

Bidmon HJ., Emde B., Oermann E., Kubitz R., Witte OW., Zilles K. Heme oxygenase-1 (HSP-32) and heme oxygenase-2 induction in neurons and glial cells of cerebral regions and its relation to iron accumulation after focal cortical photothrombosis. Exp Neurol. 168:1–22. 2001.

Blier P., de Montigny C. Current advances and trends in the treatment of depression. Trends Pharmacol Sci. 15:220–226. 1994.

Bonne O., Krausz Y., Gorfine M., Karger H., Gelfin Y., Shapira B., Chisin R., Lerer B. Cerebral hypoperfusion in medication resistant, depressed patients assessed by Tc99m HMPAO SPECT. J Affect Disord. 41:163–171. 1996.

Bonvento G., MacKenzie ET., Edvinsson L. Serotonergic innervation of the cerebral vasculature: relevance to migraine and ischaemia. Brain Res Brain Res Rev. 16:257–263. 1991.

Chiou SH., Chen SJ., Peng CH., Chang YL., Ku HH., Hsu WM., Ho LL., Lee CH. Fluoxetine up-regulates expression of cellular FLICE-inhibitory protein and inhibits LPS-induced apoptosis in hippocampus-derived neural stem cell. Biochem Biophys Res Commun. 343:391–400. 2006.

Dam M., Tonin P., De Boni A., Pizzolato G., Casson S., Ermani M., Freo U., Piron L., Battistin L. Effects of fluoxetine and maprotiline on functional recovery in post-stroke hemiplegic patients undergoing rehabilitation therapy. Stroke. 27:1211–1214. 1996.

Dawn B., Bolli R. HO-1 induction by HIF-1: a new mechanism for delayed cardioprotection? Am J Physiol Heart Circ Physiol. 289:H522–H524. 2005.

Demougeot C., Van Hoecke M., Bertrand N., Prigent-Tessier A., Mossiat C., Beley A., Marie C. Cytoprotective efficacy and mechanisms of the liposoluble iron chelator 2,2'-dipyridyl in the rat photothrombotic ischemic stroke model. J Pharmacol Exp Ther. 311:1080–1087. 2004.

Dijkhuizen RM., Ren J., Mandeville JB., Wu O., Ozdag FM., Moskowitz MA., Rosen BR., Finklestein SP. Functional magnetic resonance imaging of reorganization in rat brain after stroke. Proc Natl Acad Sci U S A. 98:12766–12771. 2001.

Duman RS., Heninger GR., Nestler EJ. A molecular and cellular theory of depression. Arch Gen Psychiatry. 54:597–606. 1997.

Fu R., Zhao ZQ., Zhao HY., Zhao JS., Zhu XL. Expression of heme oxygenase-1 protein and messenger RNA in permanent cerebral ischemia in rats. Neurol Res. 28:38–45. 2006.

Gainotti G., Antonucci G., Marra C., Paolucci S. Relation between depression after stroke, antidepressant therapy, and functional recovery. J Neurol Neurosurg Psychiatry. 71:258–261. 2001.

Geddes JW., Pettigrew LC., Holtz ML., Craddock SD., Maines MD. Permanent focal and transient global cerebral ischemia increase glial and neuronal expression of heme oxygenase-1, but not heme oxygenase-2 protein in rat brain. Neurosci Lett. 210:205–208. 1996.

Goodwin GM., de Souza RJ., Green AR. Attenuation by electroconvulsive shock and antidepressant drugs of the 5-HT1A receptor-mediated hypothermia and serotonin syndrome produced by 8-OH-DPAT in the rat. Psychopharmacology. 91:500–505. 1987.
Hanlon CA., Buffington AL., McKeown MJ. New brain networks are active after right MCA stroke when moving the ipsilesional arm. Neurology. 64:114–120. 2005.

Harukuni I., Bhardwaj A. Mechanisms of brain injury after global cerebral ischemia. Neurol Clin. 24:1–21. 2006.

Hensler JG., Kovachich GB., Frazer A. A quantitative autoradiographic study of serotonin1A receptor regulation. Effect of 5,7-dihydroxytryptamine and antidepressant treatments. Neuro-psychopharmacology. 4:131–144. 1991.
Ishii H., Bertrand N., Stanimirovic D., Strasser A., Mrsulja BB., Spatz M. The relationship between cerebral ischemic edema and monoamines: revisited. Acta Neurochir Suppl (Wien). 60:238–241. 1994.

Jakovljevic D., Tuomilehto J. Use of selective serotonin reuptake inhibitors and the risk of stroke: is there reason for concern? Stroke. 33:1448–1449. 2002.

Kim JS., Choi S., Kwon SU., Seo YS. Inability to control anger or aggression after stroke. Neurology. 58:1106–1108. 2002.

Kotila M., Numminen H., Waltimo O., Kaste M. Post-stroke depression and functional recovery in a population-based stroke register. The Finnstroke study. Eur J Neurol. 6:309–312. 1999.

Kreiss DS., Lucki I. Effects of acute and repeated administration of antidepressant drugs on extracellular levels of 5-hydroxytryptamine. J Pharmacol Exp Ther. 274:866–867. 1995.
Le Poul E., Boni C., Hanoun N., Laporte AM., Laaris N., Chauveau J., Hamon M., Lanfumey L. Differential adaption of brain 5-HT1A and 5-HT1B receptors and 5-HT transporter in rats treated chronically with fluoxetine. Neuropharmacology. 39:110–122. 2000.
Lee PJ., Jiang BH., Chin BY., Iyer NV., Alam J., Semenza GL., Choi AM. Hypoxia-inducible factor-1 mediates transcriptional activation of the heme oxygenase-1 gene in response to hypoxia. J Biol Chem. 272:5371–5381. 1997.

Li Q., Muma NA., Van de Kar LD. Chronic fluoxetine induces a gradual desensitization of 5-HT1A receptors: reductions in hypothalamic and midbrain Gi and Go proteins and in neuroendocrine responses to a 5-HT1A agonist. J Pharmacol Exp Ther. 279:1035–1042. 1996.
Maines MD. The heme oxygenase system: a regulator of second messenger gases. Annu Rev Pharmacol Toxicol. 37:517–554. 1997.
Malberg JE. Implications of adult hippocampal neurogenesis in antidepressant action. J Psychiatry Neurosci. 29:196–205. 2004.
Marks GS., Brien JF., Nakatsu K., McLaughlin BE. Does carbon monoxide have a physiological function? Trends Pharmacol Sci. 12:185–188. 1991.

Muhonen MG., Robertson SC., Gerdes JS., Loftus CM. Effects of serotonin on cerebral circulation after middle cerebral artery occlusion. J Neurosurg. 87:301–306. 1997.

Nakayama H., Jorgensen HS., Raaschou HO., Olsen TS. The influence of age on stroke outcome. The Copenhagen Stroke Study. Stroke. 25:808–813. 1994.

Nimura T., Weinstein PR., Massa SM., Panter S., Sharp FR. Heme oxygenase-1 (HO-1) protein induction in rat brain following focal ischemia. Brain Res Mol Brain Res. 37:201–208. 1996.

Otterbein LE., Choi AM. Heme oxygenase: colors of defense against cellular stress. Am J Physiol Lung Cell Mol Physiol. 279:L1029–L1037. 2000.

Panahian N., Yoshiura M., Maines MD. Overexpression of heme oxygenase-1 is neuroprotective in a model of permanent middle cerebral artery occlusion in transgenic mice. J Neurochem. 72:1187–1203. 1999.

Paolucci S., Antonucci G., Grasso MG., Morelli D., Troisi E., Coiro P., De Angelis D., Rizzi F., Bragoni M. Post-stroke depression, anti-depressant treatment and rehabilitation results. A case-control study. Cerebrovasc Dis. 12:264–271. 2001.
Parikh RM., Robinson RG., Lipsey JR., Starkstein SE., Fedoroff JP., Price TR. The impact of post-stroke depression on recovery in activities of daily living over a 2-year follow-up. Arch Neurol. 47:785–789. 1990.

Primeau F. Post-stroke depression: a critical review of the literature. Can J Psychiatry. 33:757–765. 1988.

Pulsinelli WA. Selective neuronal vulnerability: morphological and molecular characteristics. Prog Brain Res. 63:29–37. 1985.

Ramasubbu R., Patten SB. Effect of depression on stroke morbidity and mortality. Can J Psychiatry. 48:250–257. 2003.

Rampello L., Battaglia G., Raffaele R., Vecchio I., Alvano A. Is it safe to use antidepressants after a stroke? Expert Opin Drug Saf. 4:885–897. 2005.

Rasmussen A., Lunde M., Poulsen DL., S⊘rensen K., Qvitzau S., Bech P. A double-blind, placebo-controlled study of sertraline in the prevention of depression in stroke patients. Psychosomatics. 44:216–221. 2003.

Robinson RG., Schultz SK., Castillo C., Kopel T., Kosier JT., Newman RM., Curdue K., Petracca G., Starkstein SE. Nortriptyline versus fluoxetine in the treatment of depression and in short-term recovery after stroke: a placebo-controlled, double-blind study. Am J Psychiatry. 157:351–359. 2000.

Ryter SW., Otterbein LE., Morse D., Choi AM. Heme oxygenase/carbon monoxide signaling pathways: regulation and functional significance. Mol Cell Biochem. 234-235:249–263. 2002.

Sanchez V., Camarero J., Esteban B., Peter MJ., Green AR., Colado MI. The mechanisms involved in the long-lasting neuroprotective effect of fluoxetine against MDMA (‘ecstasy’)-induced degeneration of 5-HT nerve endings in rat brain. Br J Pharmacol. 134:46–57. 2001.

Schroeter M., Jander S., Stoll G. Non-invasive induction of focal cerebral ischemia in mice by photothrombosis of cortical microvessels: characterization of inflammatory responses. J Neurosci Methods. 117:43–49. 2002.

Semenza GL. Hypoxia-inducible factor 1: oxygen homeostasis and disease pathophysiology. Trends Mol Med. 7:345–350. 2001.

Semenza GL. Surviving ischemia: adaptive responses mediated by hypoxia-inducible factor 1. J Clin Invest. 106:809–812. 2000.

Starkstein SE., Robinson RG. Affective disorders and cerebral vascular disease. Br J Psychiatry. 154:170–182. 1989.

Ungvari Z., Pacher P., Kecskemeti V., Koller A. Fluoxetine dilates isolated small cerebral arteries of rats and attenuates constrictions to serotonin, norepinephrine, and a voltage-dependent Ca2+ channel opener. Stroke. 30:1949–1954. 1999.
Uyama O., Okamura N., Yanase M., Narita M., Kawabata K., Sugita M. Quantitative evaluation of vascular permeability in the gerbil brain after transient ischemia using Evans blue fluorescence. J Cereb Blood Flow Metab. 8:282–284. 1988.

Go to : 
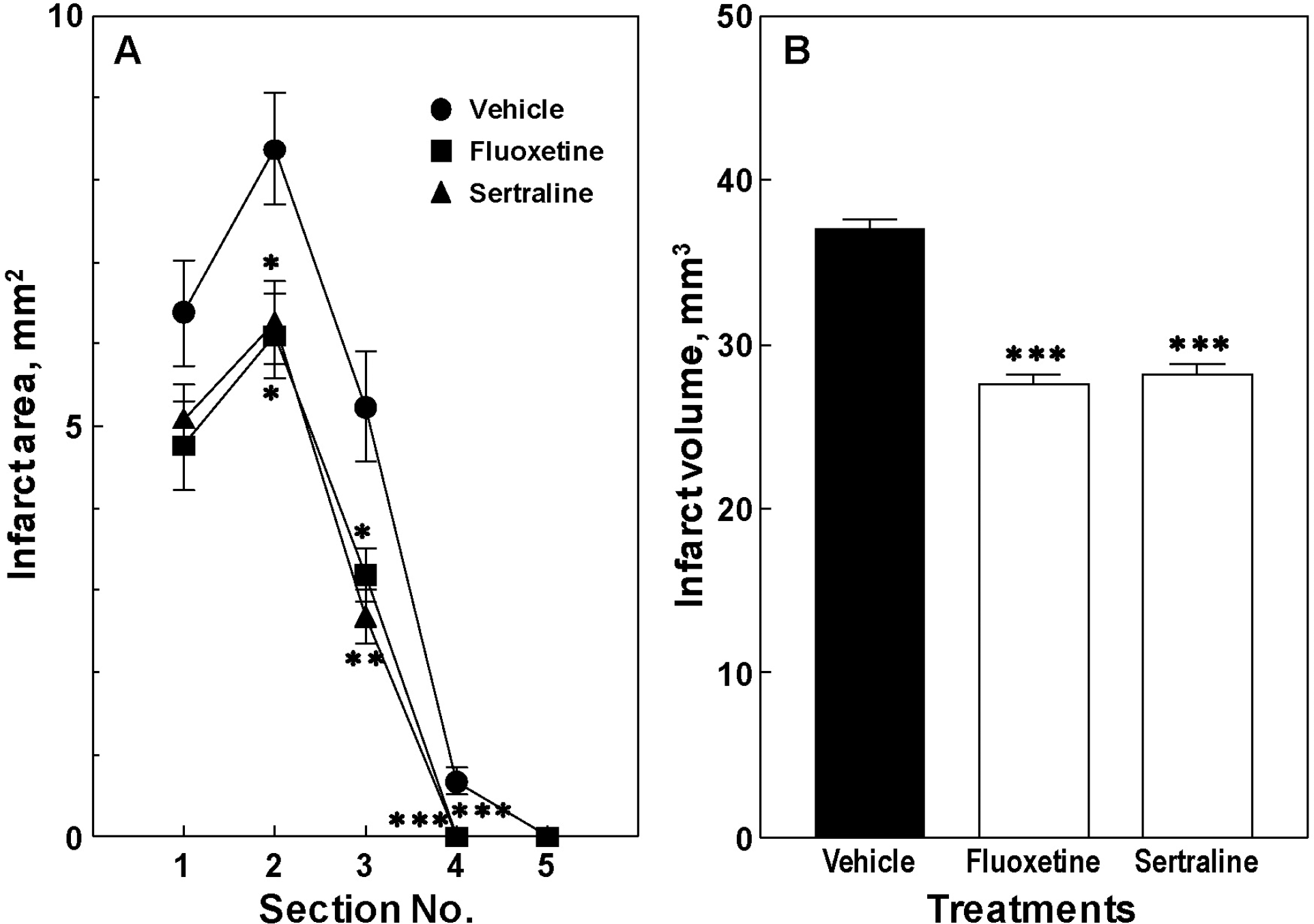 | Fig. 2.Effects of fluoxetine and sertraline on infarct area (A) and volume (B) in mice. Animals were treated daily with fluoxetine (10 mg/kg, i.p.) or sertraline (20 mg/kg, i.p.) for 14 d. Data are presented as mean±S.E.M. from 6 independent experiments. ∗p<0.05, ∗∗p<0.01, ∗∗∗p<0.001 vs. vehicle group. |
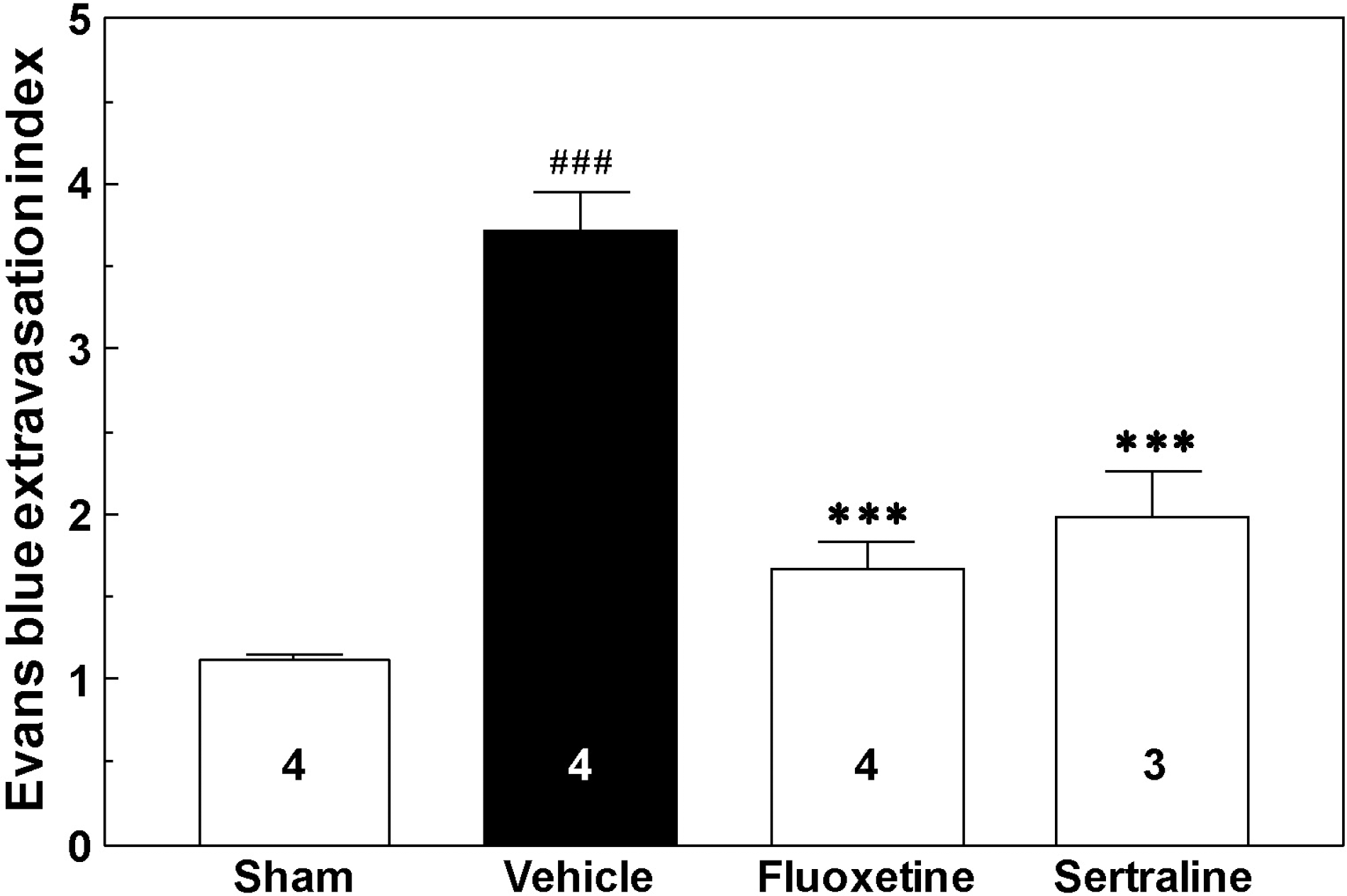 | Fig. 3.Evans blue extravasation in cerebral cortex after photothrombotic cortical ischemia in mice. Mice were sacrificed at 1 h after Evans blue injection, at 24 h after drug treatment, and at 14 d after photothrombotic cortical ischemia. The numbers in columns indicate the numbers of animals. ###p<0.001 vs. sham group. ∗∗∗p<0.001 vs. vehicle group. |
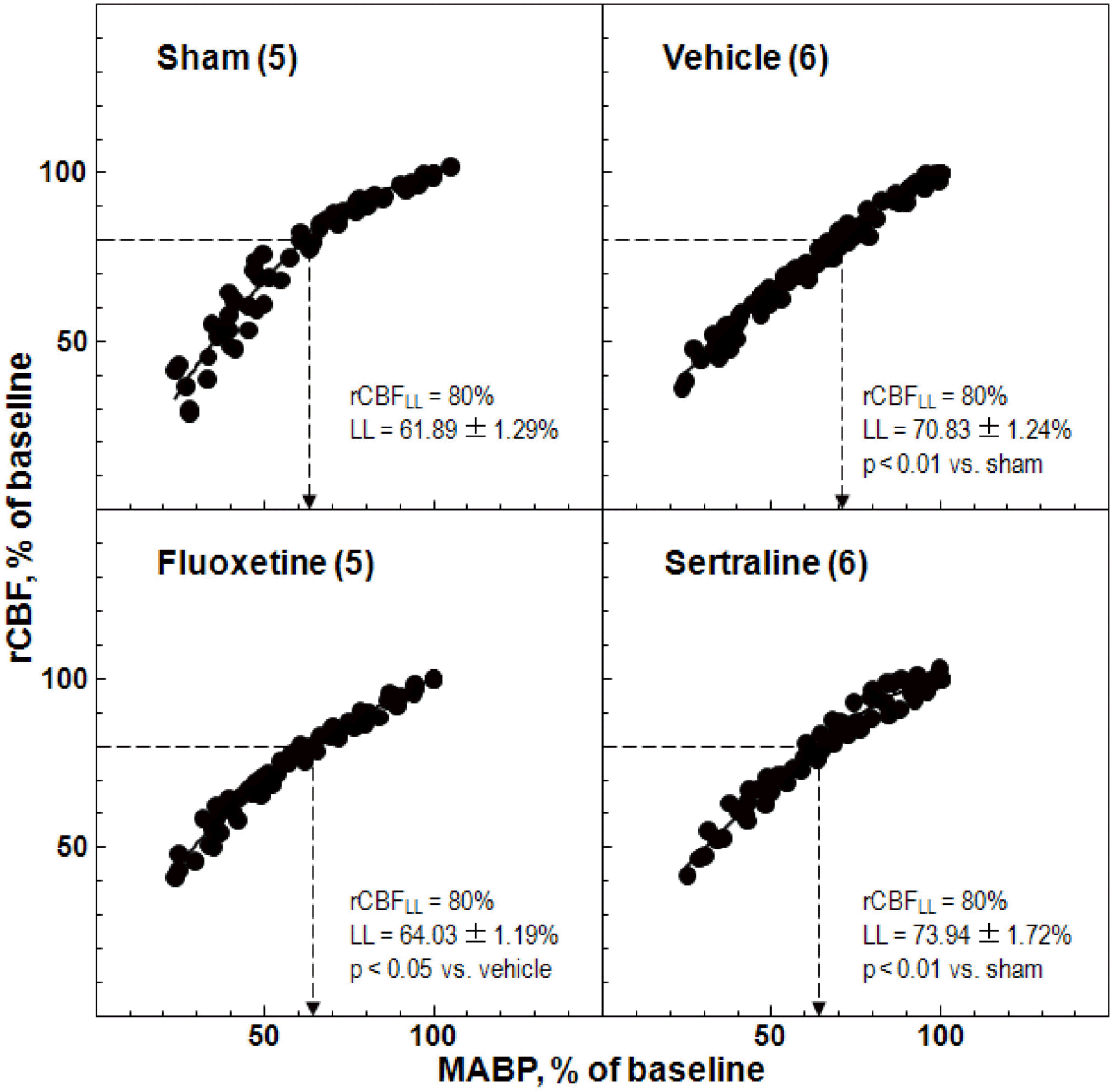 | Fig. 4.Effects of fluoxetine and sertraline on regional cerebral blood flow (rCBF) response to changes in mean arterial blood pressure (MABP). Animals were treated daily with fluoxetine (10 mg/kg, i.p.) or sertraline (20 mg/kg, i.p.) for 14 d. The numbers in parentheses indicate the numbers of animals. |
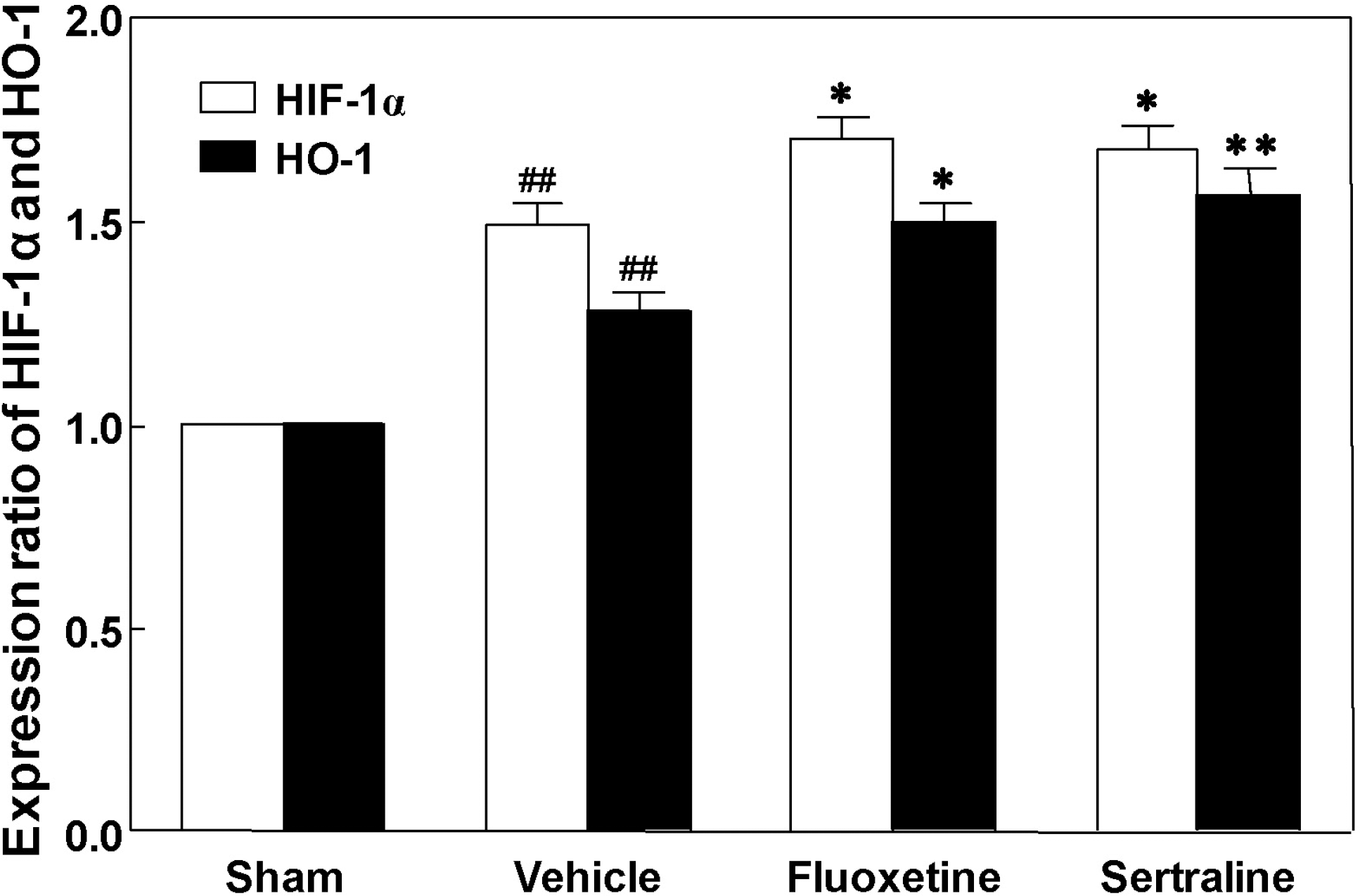 | Fig. 5.Effects of fluoxetine and sertraline on hypoxia-inducible factor-1α (HIF-1α) and heme oxygense-1 (HO-1) protein expression in photothrombotic ischemic cortex. Animals were treated daily with fluoxetine (10 mg/kg, i.p.) or sertraline (20 mg/kg, i.p.) for 14 d. Data are presented as mean±S.E.M. from 6 independent experiments. ∗p<0.05, ∗∗p<0.01 vs. vehicle, ##p<0.01 vs. sham. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download