Abstract
During operations, neurosurgeons usually perform multiple temporary occlusions of parental artery, possibly resulting in the neuronal damage. It is generally thought that neuronal damage by cerebral ischemia is associated with extracellular concentrations of the excitatory amino acids. In this study, we measured the dynamics of extracellular glutamate release in 11 vessel occlusion (VO) model to compare between single occlusion and repeated transient occlusions within short interval. Changes in cerebral blood flow were monitored by laser-Doppler flowmetry simultaneously with cortical glutamate level measured by amperometric biosensor. From real time monitoring of glutamate release in 11 VO model, the change of extracellular glutamate level in repeated transient occlusion group was smaller than that of single occlusion group, and the onset time of glutamate release in the second ischemic episode of repeated occlusion group was delayed compared to the first ischemic episode which was similar to that of single 10 min ischemic episode. These results suggested that repeated transient occlusion induces less glutamate release from neuronal cell than single occlusion, and the delayed onset time of glutamate release is attributed to endogeneous protective mechanism of ischemic tolerance.
REFERENCES
Blanco M., Lizasoain I., Sobrino T., Vivancos J., Castillo J. Ischemic preconditioning: a novel target for neuroprotective therapy. Cerebrovasc Dis. 21(Suppl 2):38–47. 2006.

Dowden J., Corbett D. Ischemic preconditioning in 18 to 20 month-old gerbils long-term survival with functional outcome measures. Stroke. 30:1240–1246. 1999.
Katayama Y., Kawamata T., Tamura T., Hovda DA., Becker DP., Tsubokawa T. Calcium-dependent glutamate release concomitant with massive potassium flux during cerebral ischemia in vivo. Brain Res. 558:136–140. 1991.

Kirino T., Tsijita Y., Tamura A. Induced tolerance to ischemia in gerbil hippocampal neurons. J Cereb Blood Flow Metab. 11:299–307. 1991.

Kitagawa K., Matsumoto M., Kuwabara K., Tagaya M., Ohtsuki T., Hata R., Ueda H., Handa N., Kimura K., Kamada T. Ischemic tolerance phenomenon detected in various brain regions. Brain Res. 561:203–211. 1991.

Lee JM., Grabb MC., Zipfel GJ., Choi DW. Brain tissue responses to ischemia. J Clin Investigat. 106:723–773. 2000.

Limbrick DD Jr. Sombati S, DeLorenzo RJ. Calcium influx constitutes the ionic basis for the maintenance of glutamate-induced extended neuronal depolarization associated with hippocampal neuronal death. Cell Calcium. 33:69–81. 2003.
Lin B., Globus MY., Dietrich WD., Busto R., Martinez E., Ginsberg MD. Differing neurochemical and morphological sequelae of global ischemia: comparison of single- and multiple- insult paradigms. J Neurochem. 59:2213–2223. 1992.
Lin CH., Chen PS., Gean PW. Glutamate preconditioning prevents neuronal death induced by combined oxygen-glucose deprivation in cultured cortical neurons. Eur J Phamacol. 589:85–93. 2008.

Nakata N., Kato H., Kogure K. Effects of repeated cerebral ischemia on extracellular amino acid concentrations measured with intracerebral microdialysis in the gerbil hippocampus. Stroke. 24:458–463. 1993.

Schaller B., Graf R. Cerebral ischemic preconditioning: an experimental phenomenon or a clinical important entity of stroke prevention? J Neurol. 249:1503–1511. 2002.
Shimazaki K., Nakamura K., Nakamura K., Oquro K., Masuzawa T., Kudo Y., Kawai N. Reduced calcium elevation in hippocampal CA1 neurons of ischemia tolerant gerbils. Neuroreport. 9:1875–1878. 1998.
Tomida S., Nowak TS Jr., Vass K., Lohr JM., Klatzo I. Experimental model for repetitive ischemic attacks in the gerbil: The cumulative effect of repeated ischemic insults. J Cereb Blood Flow Metab. 7:773–782. 1987.

Ueda Y., Obrenovitch TP., Lok SY., Sarna GS., Symon L. Changes in extracellular glutamate concentration produced in the rat striatum by repeated ischemia. Stroke. 23:1125–1131. 1992.

Zhao H., Asai S., Kanematsu K., Kunimatsu T., Khno T., Ishikawa K. Real-time monitoring of the effects of normothermia on extracellular glutamate re-uptake in the rat following global brain ischemia. Neuroreport. 8:2389–2393. 1997.
Fig. 1.
Vascular anatomy of the neck dissection at left side. A, occipital artery; B, ascending pharyngeal artery; C, superior thyroid artery; D, pterygopalatine artery; ECA, external carotid artery; CCA, common carotid artery; ICA, internal carotid artery.
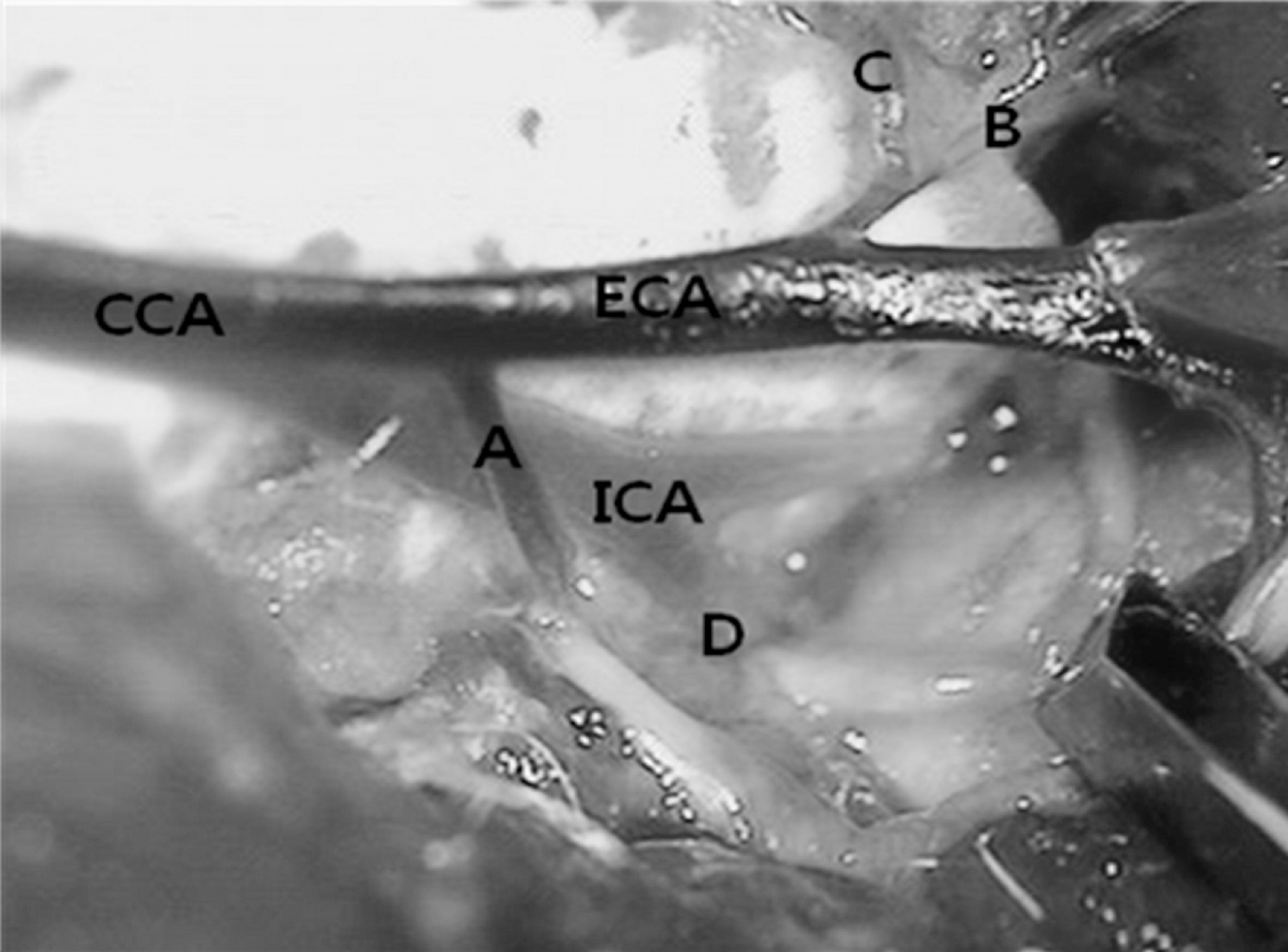
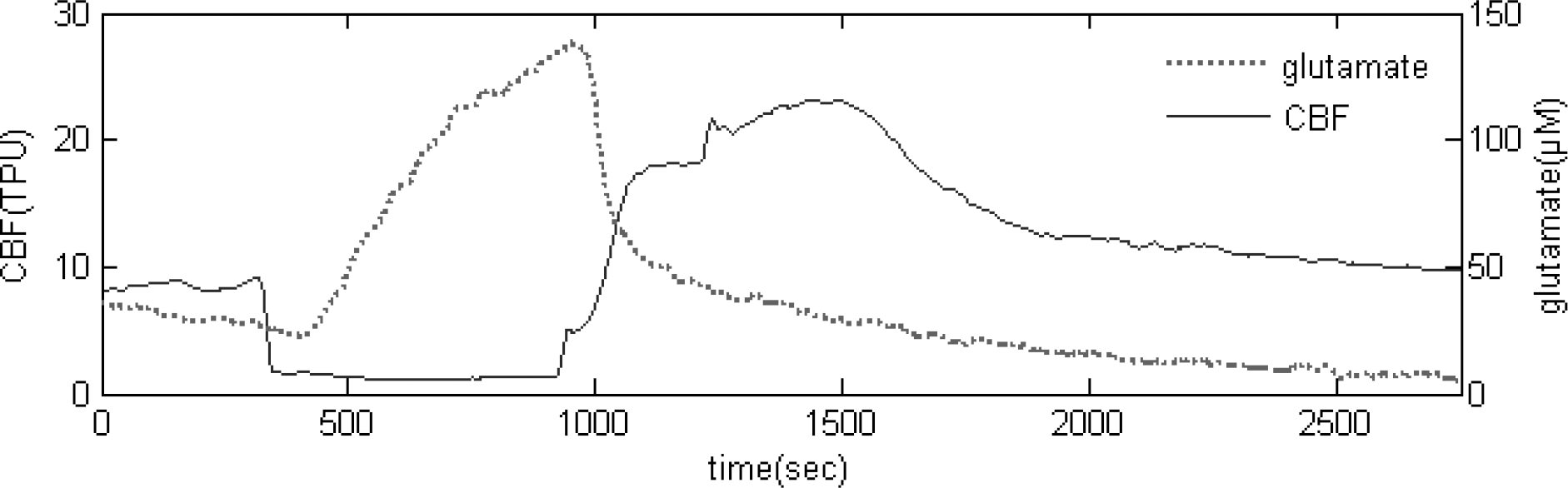
Fig. 3.
Real-time measurement of glutamate release and CBF during repeated 5 min transient ischemic episode.
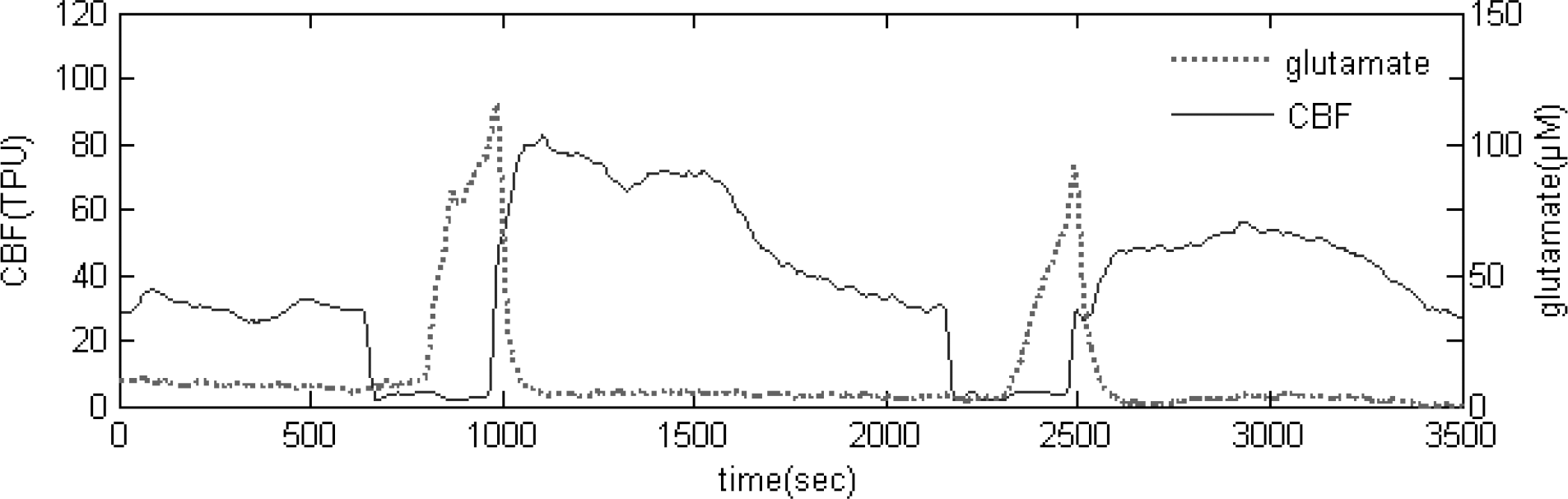
Table 1.
The changes of glutamate concentration in 11 VO method during single 10 min occlusion and repeated 5 min occlusion
| n=6 | Single occlusion group (mean±S.E.M.) | Repeated transient occlusion group | |
|---|---|---|---|
| First 5 min occlusion (mean±S.E.M.) | Second 5 min occlusion (mean±S.E.M.) | ||
| Onset time of glutamate release (sec) | 113.16±13.07 | 108.01±11.73 | 132.74±3.03∗ |
| Maximum change of glutamate release (μM) | 125.01±3.74 | 103.52±7.25∗∗ | 88.49±4.77∗∗∗ |
| Total amount of glutamate release | 38,390.44±2,252.86 | 11,021.56±2,596.68∗∗∗ | 9,689.06±2,622.65∗∗∗ |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download