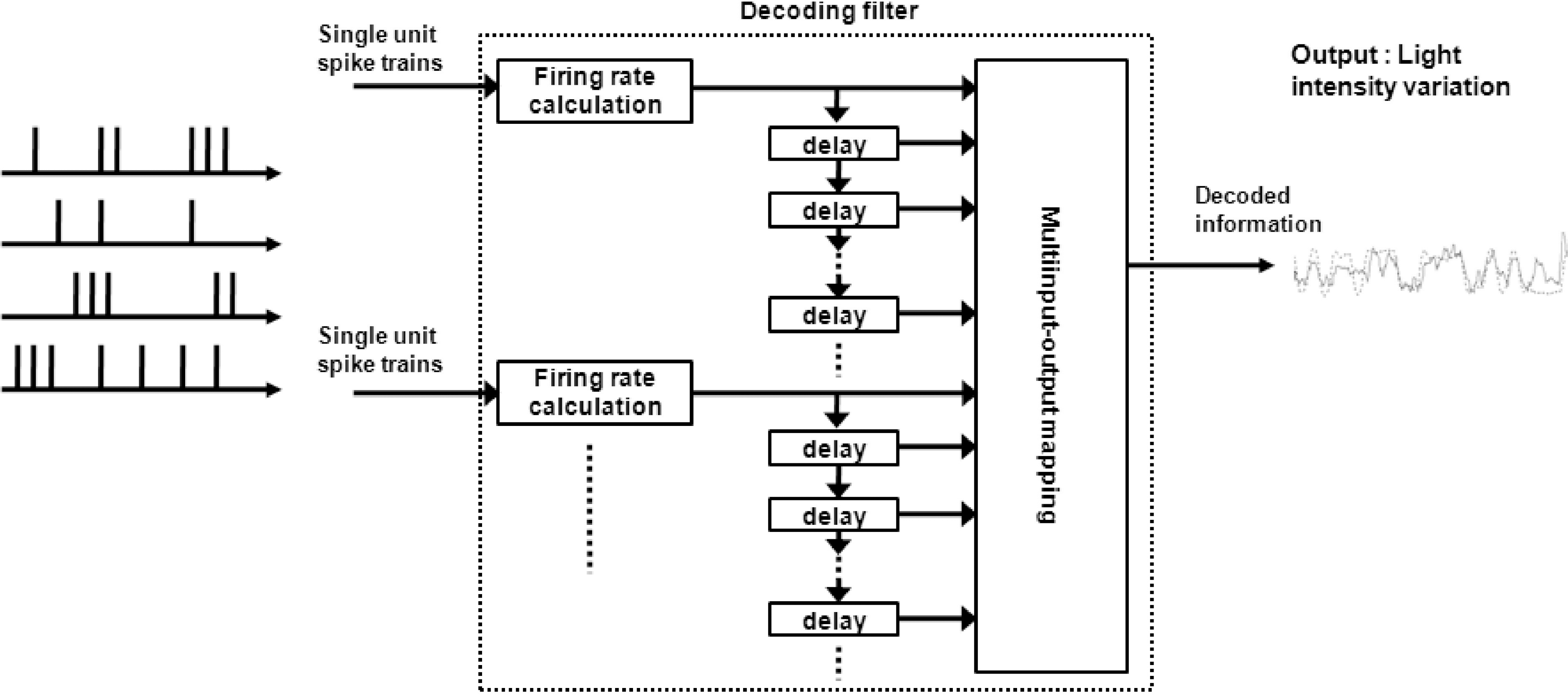
Abstract
For successful restoration of visual function by a visual neural prosthesis such as retinal implant, electrical stimulation should evoke neural responses so that the information on visual input is properly represented. A stimulation strategy, which means a method for generating stimulation waveforms based on visual input, should be developed for this purpose. We proposed to use the decoding of visual input from retinal ganglion cell (RGC) responses for the evaluation of stimulus encoding strategy. This is based on the assumption that reliable encoding of visual information in RGC responses is required to enable successful visual perception. The main purpose of this study was to determine the influence of inter-dependence among stimulated RGCs activities on decoding accuracy. Light intensity variations were decoded from multiunit RGC spike trains using an optimal linear filter. More accurate decoding was possible when different types of RGCs were used together as input. Decoding accuracy was enhanced with independently firing RGCs compared to synchronously firing RGCs. This implies that stimulation of independently-firing RGCs and RGCs of different types may be beneficial for visual function restoration by retinal prosthesis.
Go to : 
REFERENCES
Brown EN., Kass RE., Mitra PP. Multiple neural spike train data analysis: state-of-the-art and future challenges. Nat Neurosci. 7:456–461. 2004.

Brown SP., He S., Masland RH. Receptive field microstructure and dendritic geometry of retinal ganglion cells. Neuron. 27:371–383. 2000.

Cho HS., Jin GH., Goo YS. Characterization of rabbit retinal ganglion cells with multichannel recording. Korean J Med Phys. 15:228–236. 2004.
Field GD., Chichilnisky EJ. Information processing in the primate retina: circuitry and coding. Annu Rev Neurosci. 30:1–30. 2007.

Fried SI., Hsueh HA., Werblin FS. A method for generating precise temporal patterns of retinal spiking using prosthetic stimulation. J Neurophysiol. 95:970–978. 2006.

Humayun MS., Weiland JD., Fujii GY., Greenberg R., Williamson R., Little J., Mech B., Cimmarusti V., Boemel GV., Dagnelie G., de Juan E. Visual perception in a blind subject with a chronic microelectronic retinal prosthesis. Vision Res. 43:2573–2581. 2003.

Kim KH., Kim SS., Kim SJ. Superiority of nonlinear mapping in decoding multiple single-unit neuronal spike trains: a simulation study. J Neurosci Methods. 150:202–211. 2006.

Majji AB., Humayun MS., Weiland JD., Suzuki S., D'Anna SA., de Juan E. Long-term histological and electrophysiological results of an inactive epiretinal electrode array implantation in dogs. Invest Ophthalmol Vis Sci. 40:2073–2081. 1999.
Meister M., Lagnado L., Baylor DA. Concerted signaling by retinal ganglion cells. Science. 270:1207–1210. 1995.

Merabet LB., Rizzo JF., Amedi A., Somers DC., Pascual-Leone A. What blindness can tell us about seeing again: merging neuro-plasticity and neuroprosthesis. Nat Rev Neurosci. 6:71–77. 2005.
Nicolelis MAL. Methods for Neural Ensemble recording. CRC Press, Florida. 1998.
Rizzo JF., Wyatt J., Loewenstein J., Kelly S., Shire D. Methods and perceptual thresholds for short-term electrical stimulation of human retina with microelectrode arrays. Invest Ophthalmol Vis Sci. 44:5355–5361. 2003.

Ryu SB., Kim DH., Ye JH., Kim KH., Goo YS. Estimation of visual stimulus intensity from retinal ganglion cell spike trains using optimal linear filter. J Biomed Eng Res. 28:212–217. 2007.
Santos A., Humayun MS., de Juan E., Greenburg RJ., Marsh MJ., Klock IB., Milam AM. Preservation of the inner retina in retinitis pigmentosa:. a morphometric analysis. Arch Ophthal. 115:511–515. 1997.

Sekirnjak C., Hottowy P., Sher A., Dabrowski W., Litke AM., Chichilnisky EJ. Electrical stimulation of mammalian retinal ganglion cells with multielectrode array. J Neurophysiol. 95:3311–3327. 2006.
Stett A., Barth W., Weiss S., Haemmerle H., Zrenner E. Electrical multisite stimulation of the isolated chicken retina. Vision Res. 40:1785–1795. 2000.

Warland DK., Reinagel P., Meister M. Decoding visual information from a population of retinal ganglion cells. J Neurophysiol. 78:2336–2350. 1997.

Wessberg J., Stambaugh CR., Kralik JD., Beck PD., Laubach M., Chapin JK., Kim J., Biggs SJ., Srinivasan MA., Nicolelis MA. Real-time prediction of hand trajectory by ensembles of cortical neurons in primates. Nature. 408:361–365. 2000.

Go to : 
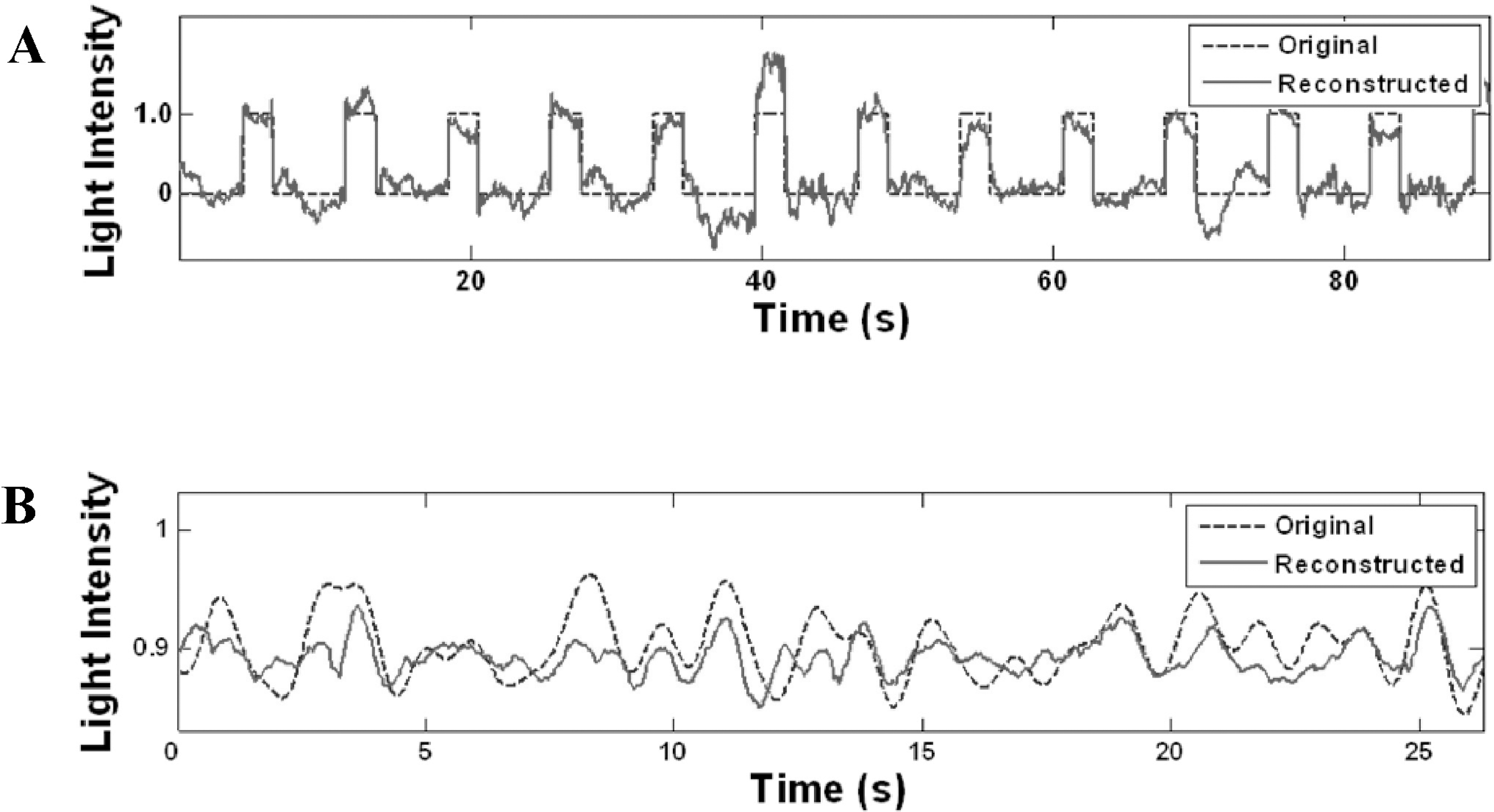 | Fig. 1.Temporal patterns of light stimulus intensity variation. Dotted: original stimulus, Solid: reconstructed stimulus by spike train decoding. (A) ON-OFF stimulus (ON: 2 s, OFF: 5 s, decoding from 4 ON RGCs), (B) Gaussian random stimulus (decoding from 5 ON RGCs). The light intensity (y axis) is represented with respect to the minimum and maximum intensity (‘0’ and ‘1’ denote the minimum and the maximum intensity levels, respectively). Frame rate was 1 Hz. |
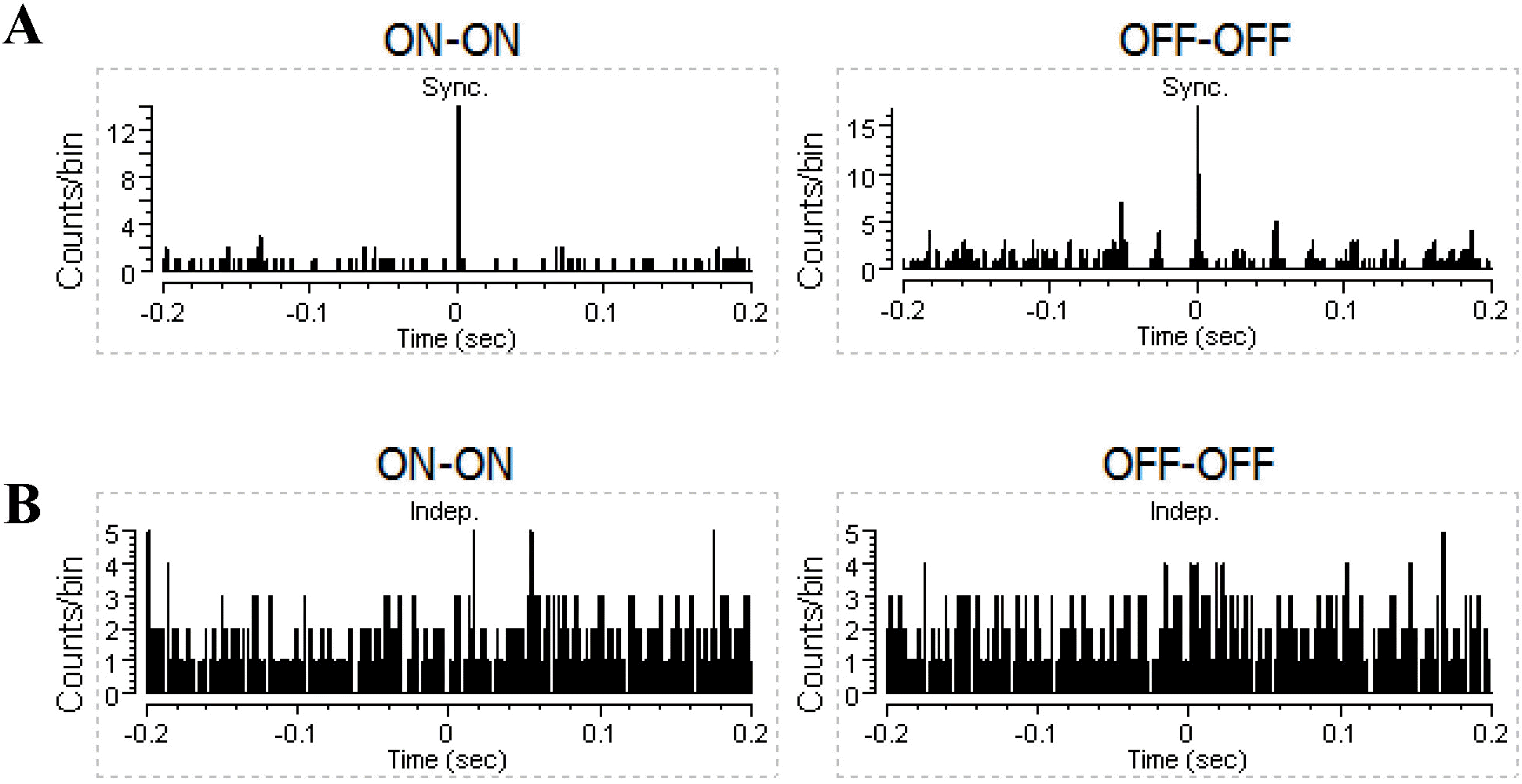 | Fig. 2.Examples of cross-correlograms for two synchronously-firing (A), and independently-firing (B) RGCs. |
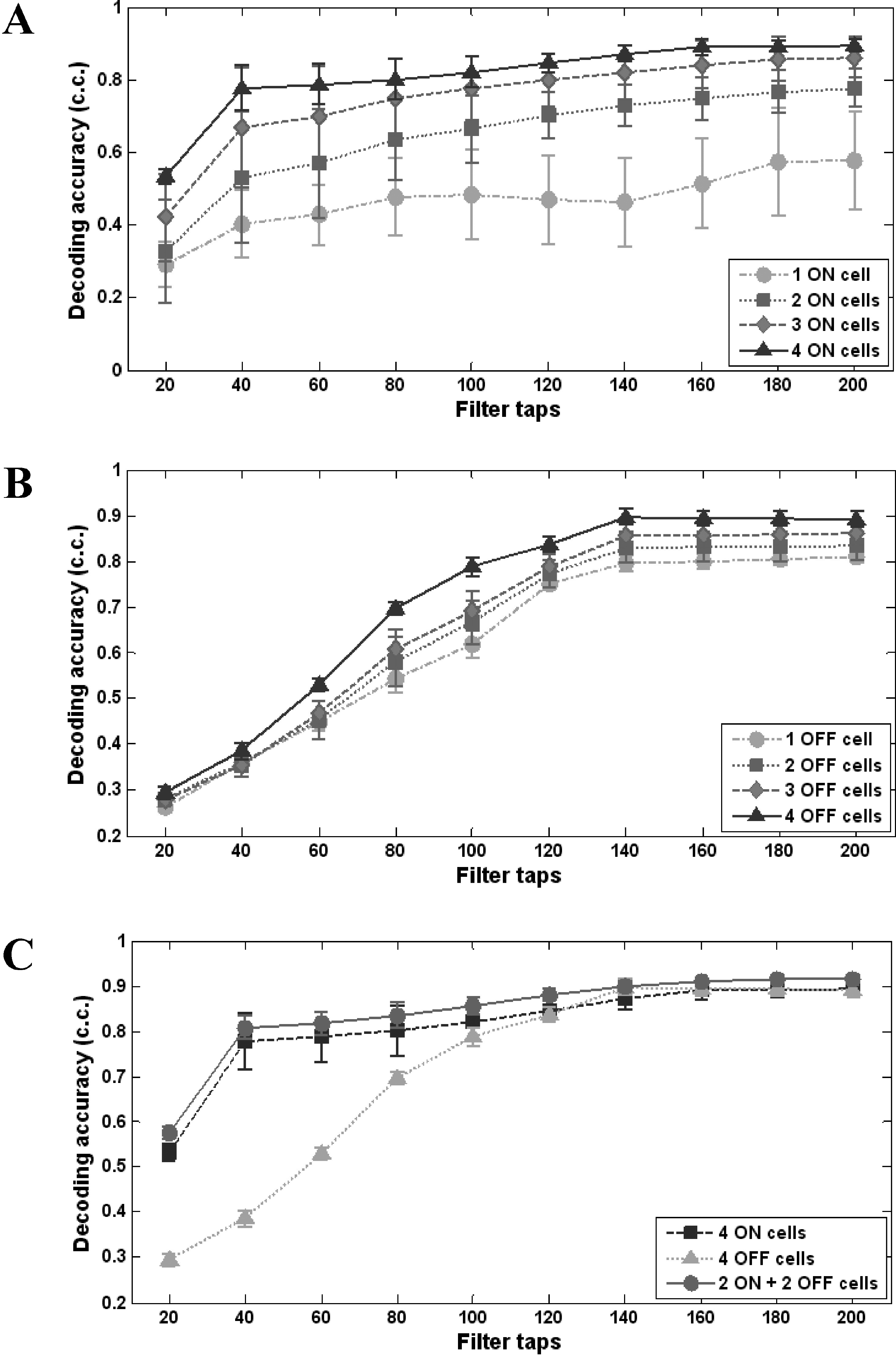 | Fig. 4.Variation of the decoding accuracy as a function of the number of filter taps. (A) ON cells (1~4 cells), (B) OFF cells (1~4 cells), (C) 2 ON cells and 2 OFF cells used together for the decoding. Each data point was obtained from 3 repeated trials of decoding from 100 s data. Error bars denote standard error. |
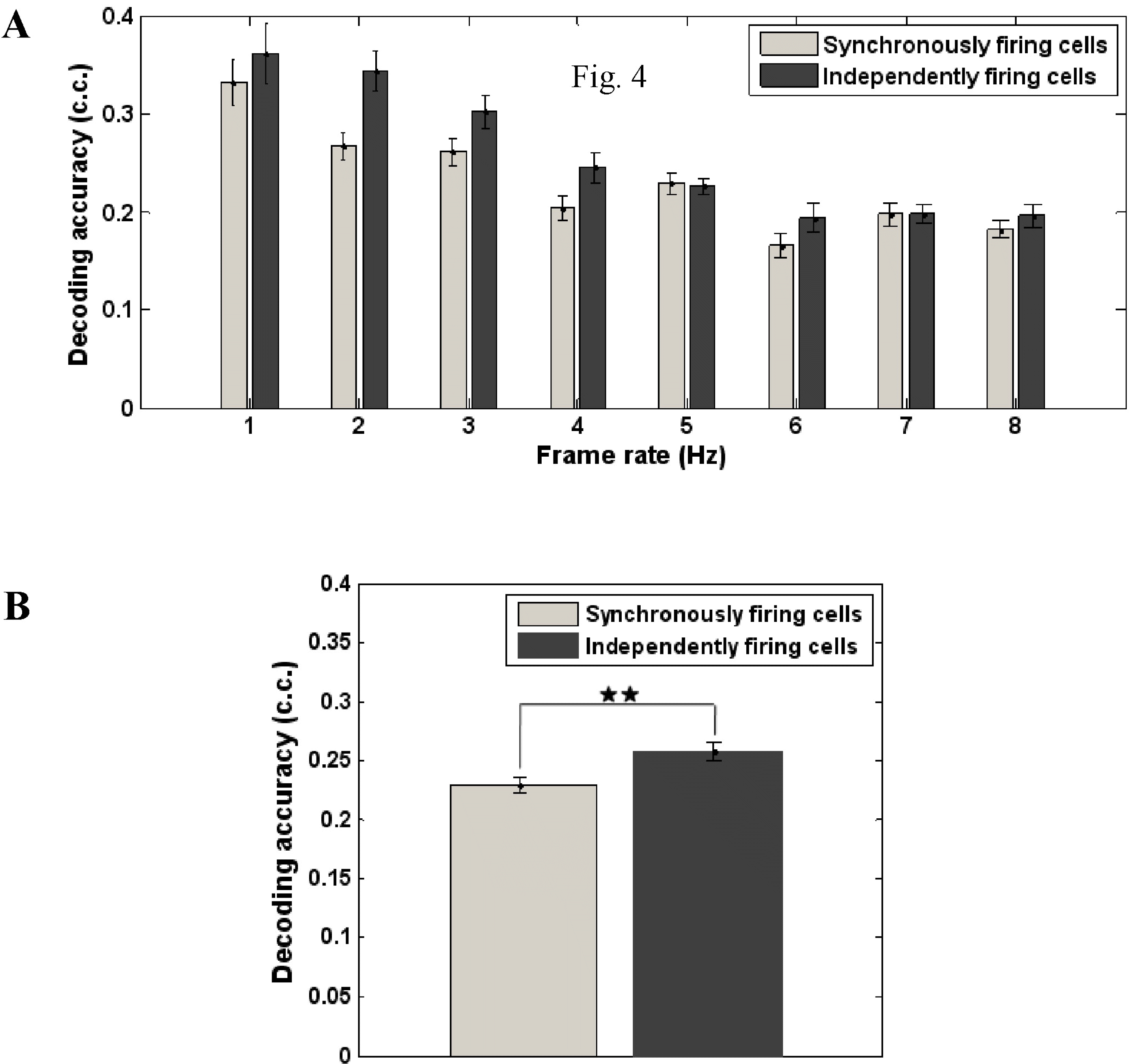 | Fig. 5.(A) Variation of the decoding accuracy as a function of frame rate, for Gaussian random stimuli obtained from 21 repeated trials (3 trials×7 cell groups), (B) Comparison of the decoding results of synchronously- firing and independently-firing RGCs, for Gaussian random stimuli (8 stimuli×3 trials×7 cell groups). |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download