Abstract
In the peripheral nerves, injury-induced cytokines and growth factors perform critical functions in the activation of both the MEK/ERK and JAK/STAT3 pathways. In this study, we determined that nerve injury-induced ERK activation was temporally correlated with STAT3 phosphorylation at the serine 727 residue. In cultured Schwann cells, we noted that ERK activation is required for the serine phosphorylation of STAT3 by neuropoietic cytokine interleukin-6 (IL-6). Serine phosphorylated STAT3 by IL-6 was transported into Schwann cell nuclei, thereby indicating that ERK may regulate the transcriptional activity of STAT3 via the induction of serine phosphorylation of STAT3. Neuregulin-1 (NRG) also induced the serine phosphorylation of STAT3 in an ERK-dependent fashion. In contrast with the IL-6 response, serine phosphorylated STAT3 induced by NRG was not detected in the nucleus, thus indicating the non-nuclear function of serine phosphorylated STAT3 in response to NRG. Finally, we determined that the inhibition of ERK prevented injury-induced serine phosphorylation of STAT3 in an ex-vivo explants culture of the sciatic nerves. Collectively, the results of this study show that ERK may be an upstream kinase for the serine phosphorylation of STAT3 induced by multiple stimuli in Schwann cells after peripheral nerve injury.
Go to : 
REFERENCES
Bleuel A., Monard D. Regulation of protease nexin-1 and angiotensin II receptor subtype 1 expression: inverse relationship in experimental models of nerve injury. J Neurosci Res. 42:562–570. 1995.

Cafferty WB., Gardiner NJ., Gavazzi I., Powell J., McMahon SB., Heath JK., Munson J., Cohen J., Thompson SW. Leukemia inhibitory factor determines the growth status of injured adult sensory neurons. J Neurosci. 21:7161–7170. 2001.

Chung J., Uchida E., Grammer TC., Blenis J. STAT3 serine phosphorylation by ERK-dependent and -independent pathways negatively modulates its tyrosine phosphorylation. Mol Cell Biol. 17:6508–6516. 1997.

Fu SY., Gordon T. The cellular and molecular basis of peripheral nerve regeneration. Mol Neurobiol. 14:67–116. 1997.

Gartsbein M., Alt A., Hashimoto K., Nakajima K., Kuroki T., Tennenbaum T. The role of protein kinase C delta activation and STAT3 Ser727 phosphorylation in insulin-induced keratinocyte proliferation. J Cell Sci. 119:470–481. 2006.
Guertin AD., Zhang DP., Mak KS., Alberta JA., Kim HA. Microanatomy of axon/glial signaling during Wallerian degeneration. J Neurosci. 25:3478–3487. 2005.

Hai M., Muja N., DeVries GH., Quarles RH., Patel PI. Comparative analysis of Schwann cell lines as model systems for myelin gene transcription studies. J Neurosci Res. 69:497–508. 2002.

Harrisingh MC., Perez-Nadales E., Parkinson DB., Malcolm DS., Mudge AW., Lloyd AC. The Ras/Raf/ERK signalling pathway drives Schwann cell dedifferentiation. EMBO J. 23:3061–3071. 2004.

Jain N., Zhang T., Fong SL., Lim CP., Cao X. Repression of Stat3 activity by activation of mitogen-activated protein kinase (MAPK). Oncogene. 17:3157–3167. 1998.

Jessen KR., Mirsky R. Negative regulation of myelination: relevance for development, injury, and demyelinating disease. Glia. 56:1552–1565. 2008.

Jessen KR., Mirsky R. The origin and development of glial cells in peripheral nerves. Nat Rev Neurosci. 6:671–682. 2005.

Jirsova K., Sodaar P., Mandys V., Bar PR. Cold jet: a method to obtain pure Schwann cell cultures without the need for cytotoxic, apoptosis-inducing drug treatment. J Neurosci Methods. 78:133–137. 1997.
Kamimura D., Ishihara K., Hirano T. IL-6 signal transduction and its physiological roles: the signal orchestration model. Rev Physiol Biochem Pharmacol. 149:1–38. 2003.

Lee HK., Seo IA., Park HK., Park YM., Ahn KJ., Yoo YH., Park HT. Nidogen is a prosurvival and promigratory factor for adult Schwann cells. J Neurochem. 102:686–698. 2007.

Lee HK., Seo IA., Suh DK., Hong JI., Yoo YH., Park HT. Interleukin-6 is required for the early induction of glial fibrillary acidic protein in Schwann cells during Wallerian degeneration. J Neurochem. 108:776–786. 2009.

Lee N., Neitzel KL., Devlin BK., MacLennan AJ. STAT3 phosphorylation in injured axons before sensory and motor neuron nuclei: potential role for STAT3 as a retrograde signaling transcription factor. J Comp Neurol. 474:535–545. 2004.

Monje PV., Bartlett Bunge M., Wood PM. Cyclic AMP synergistically enhances neuregulin-dependent ERK and Akt activation and cell cycle progression in Schwann cells. Glia. 53:649–659. 2006.

Ng DC., Lin BH., Lim CP., Huang G., Zhang T., Poli V., Cao X. Stat3 regulates microtubules by antagonizing the depolymerization activity of stathmin. J Cell Biol. 172:245–257. 2006a.

Ng YP., Cheung ZH., Ip NY. STAT3 as a downstream mediator of Trk signaling and functions. J Biol Chem. 281:15636–15644. 2006b.

Plaza-Menacho I., van der Sluis T., Hollema H., Gimm O., Buys CH., Magee AI., Isacke CM., Hofstra RM., Eggen BJ. Ras/ERK1/2-mediated STAT3 Ser727 phosphorylation by familial medullary thyroid carcinoma-associated RET mutants induces full activation of STAT3 and is required for c-fos promoter activation, cell mitogenicity, and transformation. J Biol Chem. 282:6415–6424. 2007.

Qiu J., Cafferty WB., McMahon SB., Thompson SW. Conditioning injury-induced spinal axon regeneration requires signal transducer and activator of transcription 3 activation. J Neurosci. 25:1645–1653. 2005.

Sheu JY., Kulhanek DJ., Eckenstein FP. Differential patterns of ERK and STAT3 phosphorylation after sciatic nerve transection in the rat. Exp Neurol. 166:392–402. 2000.

Shi X., Zhang H., Paddon H., Lee G., Cao X., Pelech S. Phosphorylation of STAT3 serine-727 by cyclin-dependent kinase 1 is critical for nocodazole-induced mitotic arrest. Biochemistry. 45:5857–5867. 2006.

Thomson CE., Griffiths IR., McCulloch MC., Kyriakides E., Barrie JA., Montague P. In vitro studies of axonally-regulated Schwann cell genes during Wallerian degeneration. J Neurocytol. 22:590–602. 1993.

Wen Z., Zhong Z., Darnell JE Jr. Maximal activation of transcription by Stat1 and Stat3 requires both tyrosine and serine phosphorylation. Cell. 82:241–250. 1995.
Go to : 
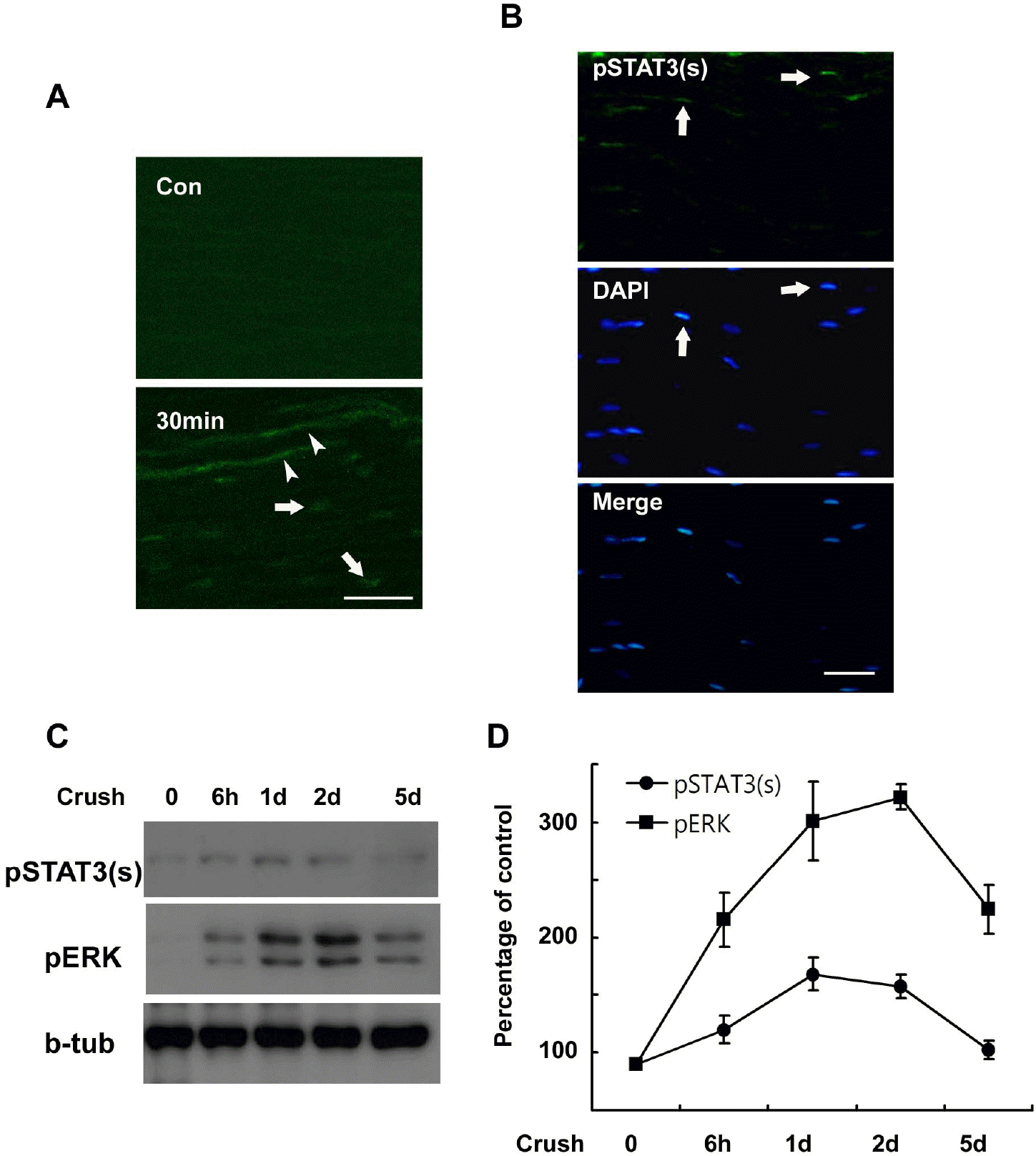 | Fig. 1.Serine phosphorylation of STAT3 in the sciatic nerve following crush injury. (A, B) Frozen sections from control and injured sciatic nerves were immunostained with an antibody against serine phosphorylated STAT3 (pSTAT3(s)). Axons (arrowheads) and nuclei of SC-like cells (arrows) display a rapid induction in the serine phosphorylation of STAT3 at 30 min (A) and 60 min (B) after lesion. Scale bar; 30 μm. (C) Protein lysates were prepared from the injured sciatic nerves and analyzed with Western blot analysis for detection of serine phosphorylated STAT3 and pERK. (D) A quantitative analysis for result (C). |
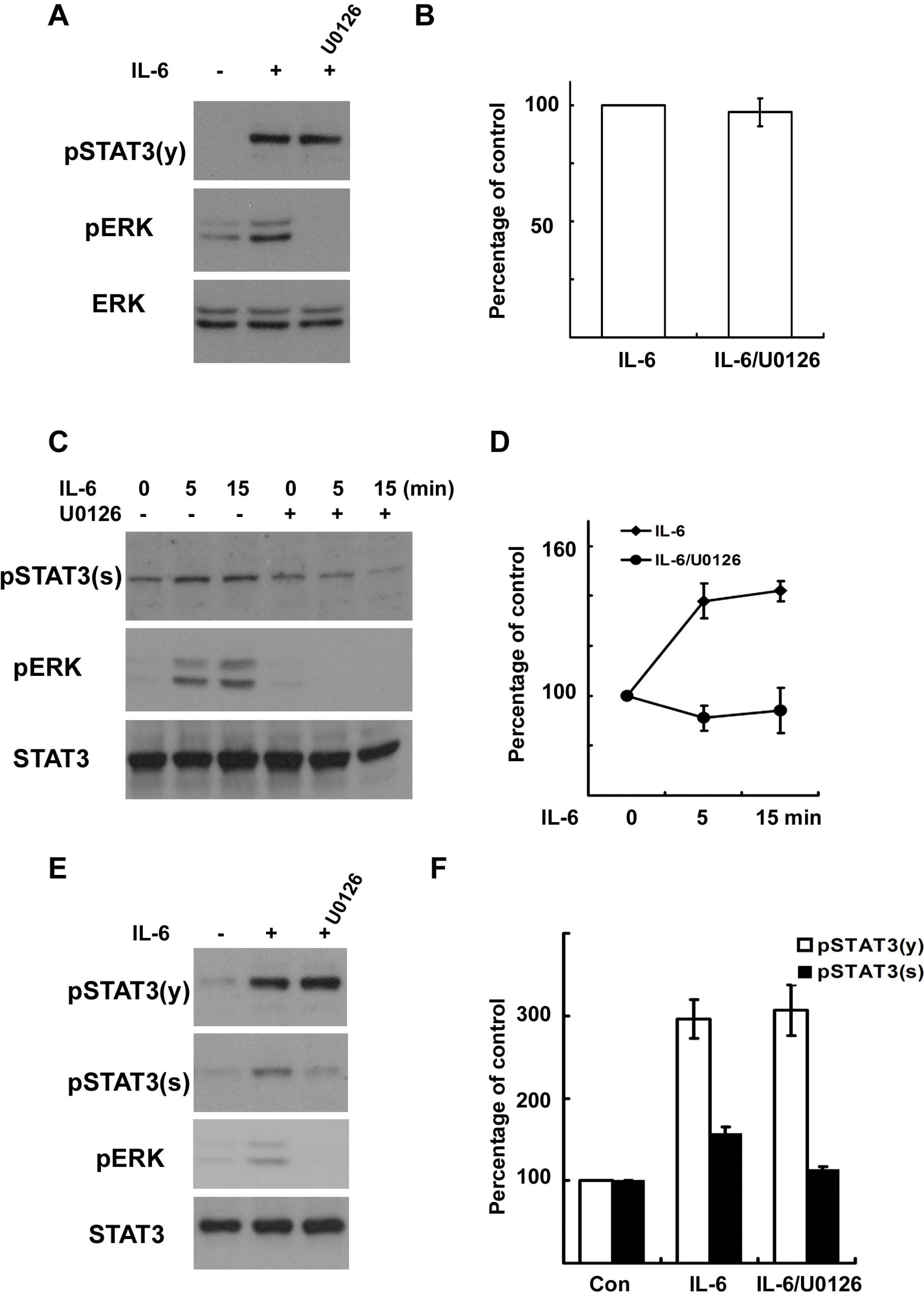 | Fig. 2.IL-6 induces serine phosphorylation of STAT3 in Schwann cells. RT4 schwannoma cells (A~D) and primary SCs (E, F) were serum starved overnight and then treated with IL-6 (50 ng/ml) for the indicated times. Total cell lysates were subjected to Western blot analysis using antibodies specific for phosphorylated proteins. (A, B) RT4 cells were treated with IL-6 for 15 min, and the cellular lysates were analyzed using the indicated antibodies. (B) A quantitative analysis of tyrosine phosphorylated STAT3 in result (A). (C, D) RT4 cells were treated with IL-6 for the indicated time in the presence or absence of U0126. (D) A quantitative analysis of serine phosphorylated STAT3 in result (C). (E, F) Primary SCs were treated with IL-6 for 15 min, and the total lysates were analyzed by Western blot analysis using the indicated antibodies. (F) A quantitative analysis of phosphorylated STAT3 in result (E). |
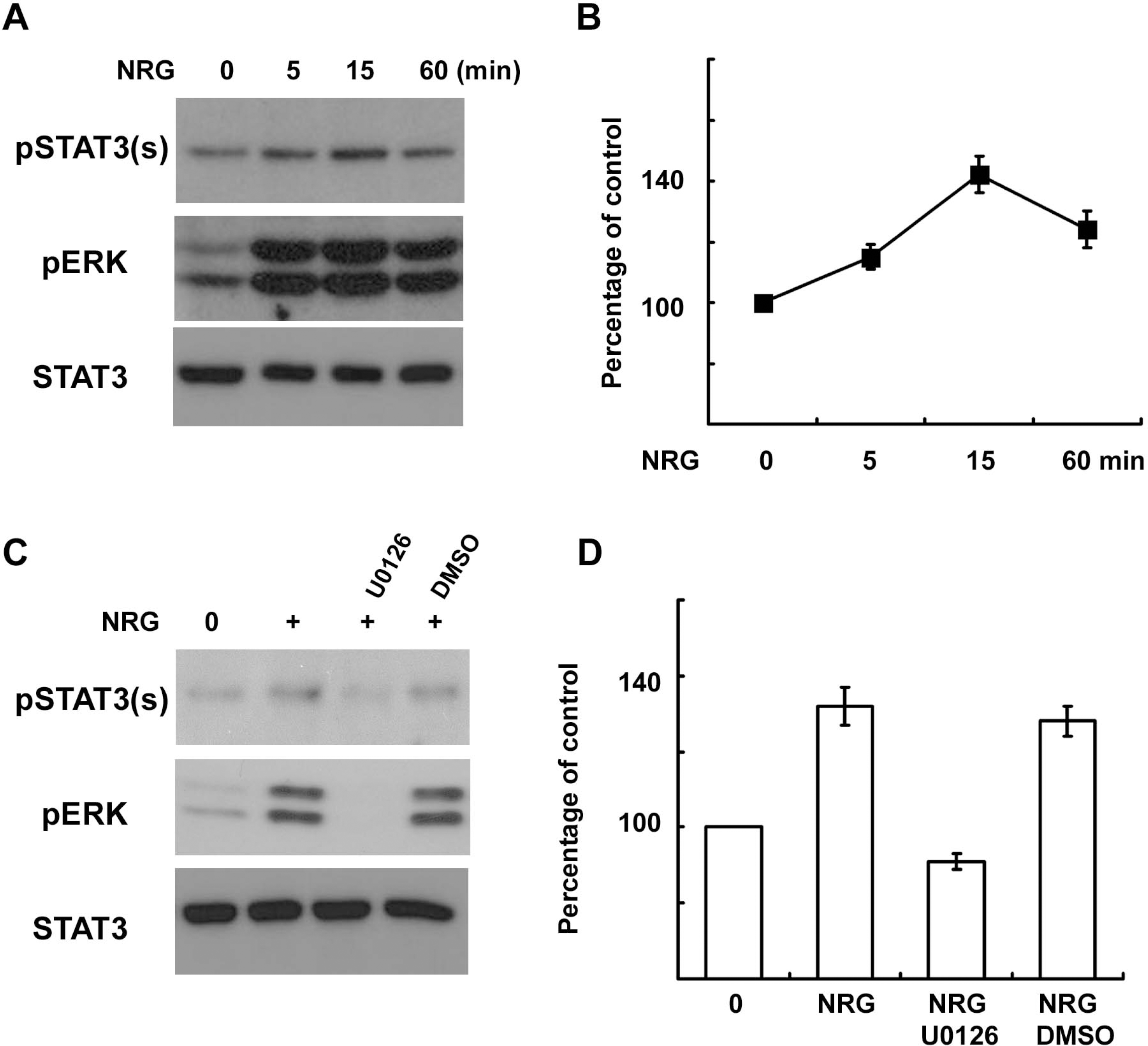 | Fig. 3.NRG-1 induces serine phosphorylation of STAT3 in Schwann cells. (A, B) Primary SCs were serum starved overnight and then treated with NRG-1 (200 ng/ml) for the indicated times. Total cell lysates were subjected to Western blot analysis using antibodies specific for phosphorylated proteins. (B) A quantitative analysis of serine phosphorylated STAT3 in result (A). (C, D) Primary SCs were treated with NRG-1 for 15 min in the presence or absence of U0126 or DMSO, and cellular lysates were analyzed with Western blot analysis. (D) A quantitative analysis of serine phosphory-lated STAT3 in result (C). |
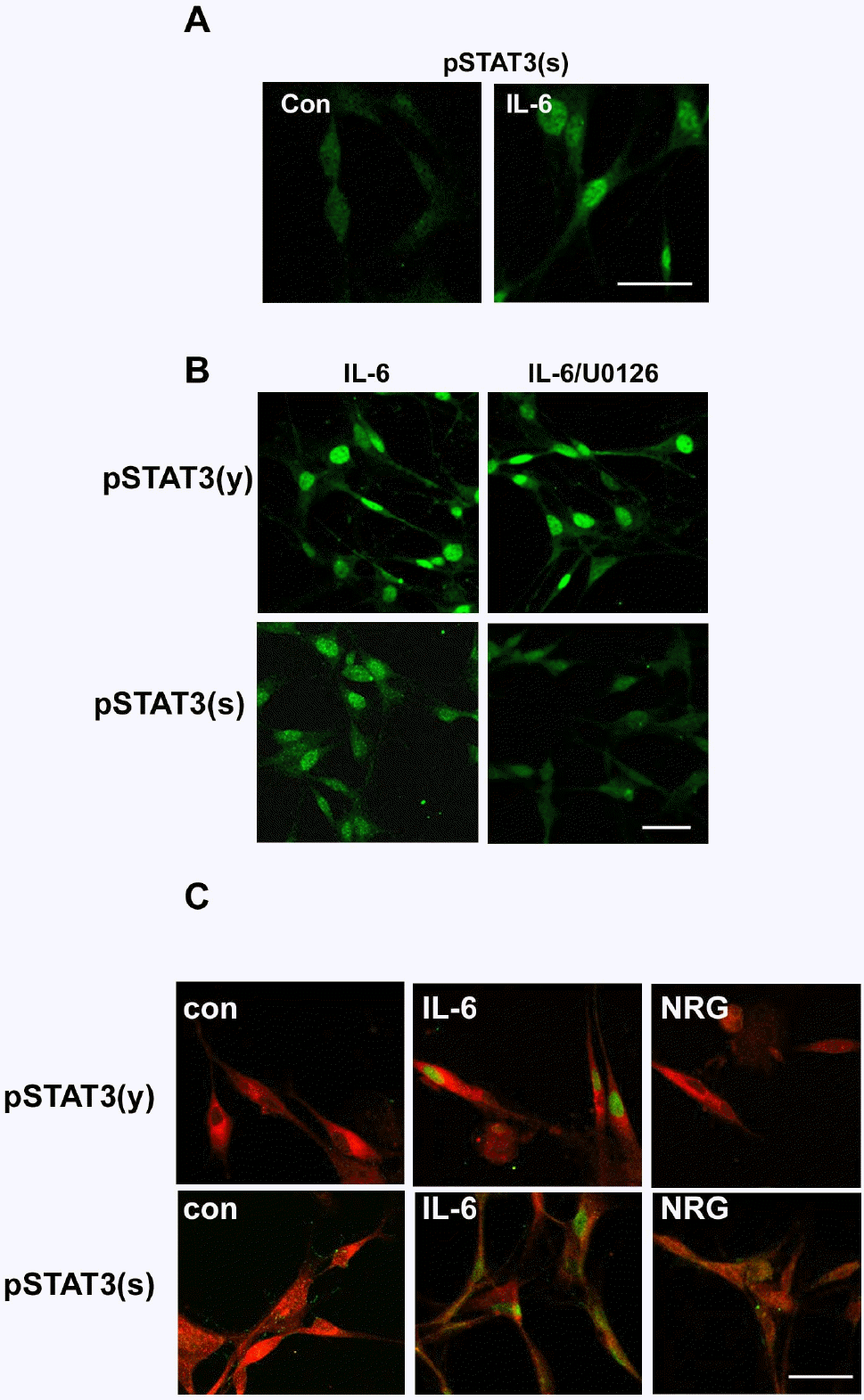 | Fig. 4.Differential localization of phosphorylated STAT3 by IL-6 and NRG-1. (A) After 20 min of stimulation with IL-6, RT4 cells were fixed and immunostained for serine phosphorylated STAT3. Con; untreated control. (B) RT4 cells were pretreated with U0126 for 30 min and then stimulated with IL-6 for 20 min. Cells were immunostained for tyrosine or serine phosphorylated STAT3. Scale bar; 30 μm. (C) Primary SCs were treated with IL-6 or NRG for 20 min, and the cells were fixed and double immunostained for a SC marker (S100) and phosphorylated STAT3. Scale bar; 30 μm. |
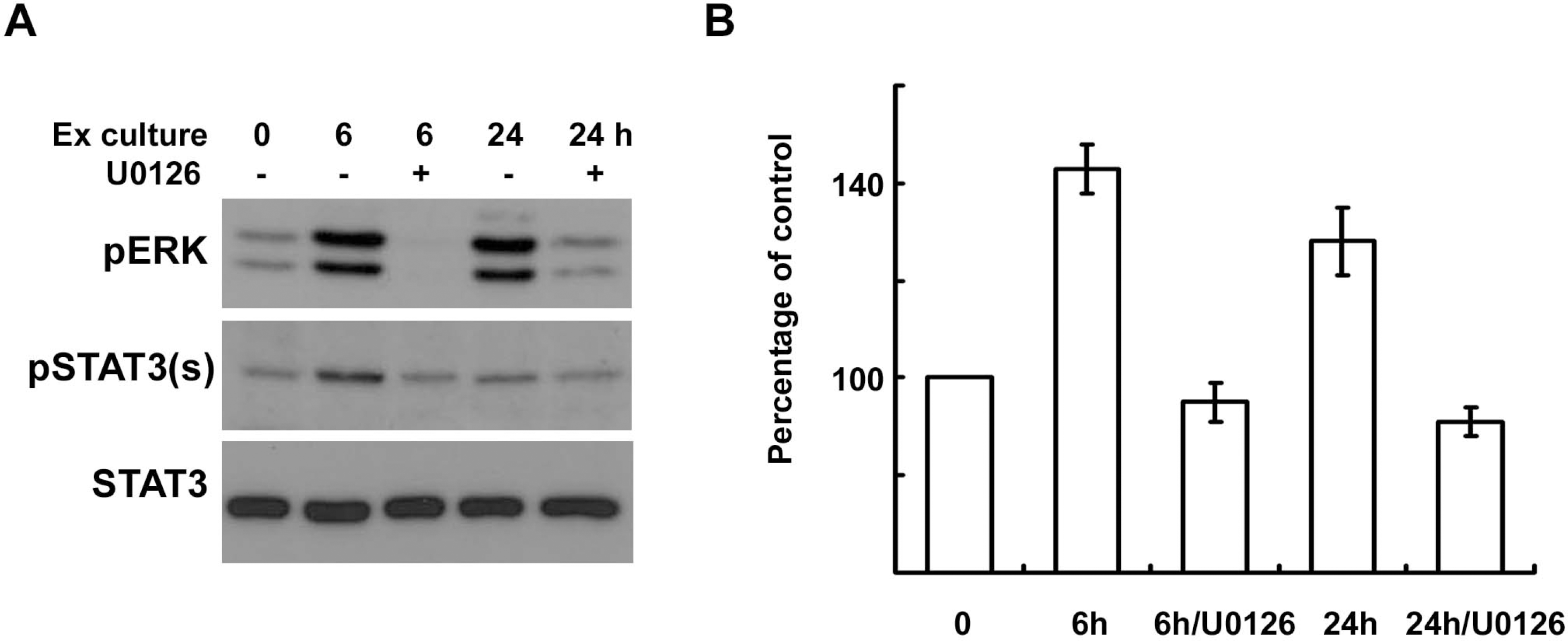 | Fig. 5.ERK inhibition reduces the serine phosphorylation of STAT3 in explant cultures of sciatic nerves. (A, B) Sciatic nerve explants were cultured for 6 or 24 h in the presence or absence of U0126, and then the protein lysates were analyzed with Western blot analysis using an antibody against serine phosphorylated STAT3 and anti-pERK antibody. (B) A quantitative analysis of serine phosphorylated STAT3 in result (A). |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download