Abstract
We investigated whether deficiency of inducible nitric oxide synthase (iNOS) could prevent isoproterenol-induced cardiac hypertrophy in iNOS knockout (KO) mice. Isoproterenol was continuously infused subcutaneously (15 mg/kg/day) using an osmotic minipump. Isoproterenol reduced body weight and fat mass in both iNOS KO and wild-type mice compared with saline-infused wild-type mice. Isoproterenol increased the heart weight in both iNOS KO and wild-type mice but there was no difference between iNOS KO and wild-type mice. Posterior wall thickness of left ventricle showed the same tendency with heart weight. Protein level of iNOS in the left ventricle was increased in isoproterenol-infused wild-type mice. The gene expression of interleukin-6 (IL-6) and transforming growth factor-β (TGF-β) in isoproterenol-infused wild-type was measured at 2, 4, 24, and 48-hour and isoproterenol increased both IL-6 (2, 4, 24, and 48-hour) and TGF-β (4 and 24-hour). Isoproterenol infusion for 7 days increased the mRNA level of IL-6 and TGF-β in iNOS KO mice, whereas the gene expression in wild-type mice was not increased. Phosphorylated form of extracellular signal-regulated kinases (pERK) was also increased by isoproterenol at 2 and 4-hour but was not increased at 7 days after infusion in wild-type mice. However, the increased pERK level in iNOS KO mice was maintained even at 7 days after isoproterenol infusion. These results suggest that deficiency of iNOS does not prevent isoproterenol-induced cardiac hypertrophy and may have potentially harmful effects on cardiac hypertrophy.
Go to : 
REFERENCES
Barry SP., Davidson SM., Townsend PA. Molecular regulation of cardiac hypertrophy. Int J Biochem Cell Biol. 40:2023–2039. 2008.

Boluyt MO., O'Neill L., Meredith AL., Bing OH., Brooks WW., Conrad CH., Crow MT., Lakatta EG. Alterations in cardiac gene expression during the transition from stable hypertrophy to heart failure. Marked upregulation of genes encoding extracellular matrix components. Circ Res. 75:23–32. 1994.

Bueno OF., Molkentin JD. Involvement of extracellular signal-regulated kinases 1/2 in cardiac hypertrophy and cell death. Circ Res. 91:776–781. 2002.

Cha H. The Effect of iNOS deficiency on age-associated insulin resistance. MS Thesis. 2009.
Cmungrue IN., Gros R., You X., Pirani A., Azad A., Csont T., Schulz R., Butany J., Stewart DJ., Husain M. Cardiomyocyte over-expression of iNOS in mice results in peroxynitrite generation, heart block, and sudden death. J Clin Invest. 109:735–743. 2002.
Collins S., Surwit RS. The beta-adrenergic receptors and the control of adipose tissue metabolism and thermogenesis. Recent Prog Horm Res. 56:309–328. 2001.
Funakoshi H., Kubota T., Kawamura N., Machida Y., Feldman AM., Tsutsui H., Shimokawa H., Takeshita A. Disruption of inducible nitric oxide synthase improves beta-adrenergic inotropic responsiveness but not the survival of mice with cytokine-induced cardiomyopathy. Circ Res. 90:959–965. 2002.
Gardner DG., Chen S., Glenn DJ., Grigsby CL. Molecular biology of the natriuretic peptide system: implications for physiology and hypertension. Hypertension. 49:419–426. 2007.
Godecke A., Molojavyi A., Heger J., Flogel U., Ding Z., Jacoby C., Schrader J. Myoglobin protects the heart from inducible nitric-oxide synthase (iNOS)-mediated nitrosative stress. J Biol Chem. 278:21761–21766. 2003.
Greenwood JP., Scott EM., Stoker JB., Mary DA. Hypertensive left ventricular hypertrophy: relation to peripheral sympathetic drive. J Am Coll Cardiol. 38:1711–1717. 2001.

IKodama H., Fukuda K., Pan J., Sano M., Takahashi T., Kato T., Makino S., Manabe T., Murata M., Ogawa S. Significance of ERK cascade compared with JAK/STAT and PI3-K pathway in gp130-mediated cardiac hypertrophy. Am J Physiol Heart Circ Physiol. 279:H1635–1644. 2000.
Imamura G., Bertelli AA., Bertelli A., Otani H., Maulik N., Das DK. Pharmacological preconditioning with resveratrol: an insight with iNOS knockout mice. Am J Physiol Heart Circ Physiol. 282:H1996–2003. 2002.
Ji K., Minakawa M., Fukui K., Suzuki Y., Fukuda I. Increased superoxide radical with a decrease in vascular endothelial growth factor and inducible nitric oxide synthase level leads to the progression of left ventricular hypertrophy in a pressure-overload rat heart model. Ann Thorac Cardiovasc Surg. 14:210–217. 2008.
Krenek P., Kmecova J., Kucerova D., Bajuszova Z., Musil P., Gazova A., Ochodnicky P., Klimas J., Kyselovic J. Isoproterenol-induced heart failure in the rat is associated with nitric oxide-dependent functional alterations of cardiac function. Eur J Heart Fail. 11:140–146. 2009.

Kundu S., Kumar M., Sen U., Mishra PK., Tyagi N., Metreveli N., Lominadze D., Rodriguez W., Tyagi SC. Nitrotyrosinylation, remodeling and endothelial-myocyte uncoupling in iNOS, cystathionine beta synthase (CBS) knockouts and iNOS/CBS double knockout mice. J Cell Biochem. 106:119–126. 2009.

Levy D., Garrison RJ., Savage DD., Kannel WB., Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the framingham heart study. N Engl J Med. 322:1561–1566. 1990.

Li RK., Li G., Mickle DA., Weisel RD., Merante F., Luss H., Rao V., Christakis GT., Williams WG. Overexpression of transforming growth factor-beta1 and insulin-like growth factor-I in patients with idiopathic hypertrophic cardiomyopathy. Circulation. 96:874–881. 1997.
Mikaelian I., Coluccio D., Morgan KT., Johnson T., Ryan AL., Rasmussen E., Nicklaus R., Kanwal C., Hilton H., Frank K., Fritzky L., Wheeldon EB. Temporal gene expression profiling indicates early up-regulation of interleukin-6 in isoproterenol-induced myocardial necrosis in rat. Toxicol Pathol. 36:256–264. 2008.

Osadchii OE. Cardiac hypertrophy induced by sustained beta-adrenoreceptor activation: pathophysiological aspects. Heart Fail Rev. 12:66–86. 2007.
Pauschinger M., Knopf D., Petschauer S., Doerner A., Poller W., Schwimmbeck PL., Kuhl U., Schultheiss HP. Dilated cardiomyopathy is associated with significant changes in collagen type I/III ratio. Circulation. 99:2750–2756. 1999.

Pelat M., Verwaerde P., Galitzky J., Lafontan M., Berlan M., Senard JM., Montastruc JL. High isoproterenol doses are required to activate beta3-adrenoceptor-mediated functions in dogs. J Pharmacol Exp Ther. 304:246–253. 2003.
Rajabi M., Kassiotis C., Razeghi P., Taegtmeyer H. Return to the fetal gene program protects the stressed heart: a strong hypothesis. Heart Fail Rev. 12:331–343. 2007.

Schlaich MP., Kaye DM., Lambert E., Sommerville M., Socratous F., Esler MD. Relation between cardiac sympathetic activity and hypertensive left ventricular hypertrophy. Circulation. 108:560–565. 2003.

Strand AH., Gudmundsdottir H., Os I., Smith G., Westheim AS., Bjornerheim R., Kjeldsen SE. Arterial plasma noradrenaline predicts left ventricular mass independently of blood pressure and body build in men who develop hypertension over 20 years. J Hypertens. 24:905–913. 2006.

Szabo J., Csaky L., Szegi J. Experimental cardiac hypertrophy induced by isoproterenol in the rat. Acta Physiol Acad Sci Hung. 46:281–285. 1975.
Tanaka T., Kanda T., Itoh T., Tsugawa H., Takekoshi N., Yamakawa J., Kurimoto M., Kurabayashi M. Increased cardiac weight in interleukin-6 transgenic mice with viral infection accompanies impaired expression of natriuretic peptide genes. Res Commun Mol Pathol Pharmacol. 110:275–283. 2001.
Tsuchiya K., Sakai H., Suzuki N., Iwashima F., Yoshimoto T., Shichiri M., Hirata Y. Chronic blockade of nitric oxide synthesis reduces adiposity and improves insulin resistance in high fat-induced obese mice. Endocrinology. 148:4548–4556. 2007.

Villarreal FJ., Dillmann WH. Cardiac hypertrophy-induced changes in mRNA levels for TGF-beta 1, fibronectin, and collagen. Am J Physiol. 262:H1861–1866. 1992.

Wang T., Yan M., Li J., Zheng X. The role of iNOS-derived NO in the antihypertrophic actions of B-type natriuretic peptide in neonatal rat cardiomyocytes. Mol Cell Biochem. 302:169–177. 2007.

Xi L., Jarrett NC., Hess ML., Kukreja RC. Essential role of inducible nitric oxide synthase in monophosphoryl lipid A-induced late cardioprotection: evidence from pharmacological inhibition and gene knockout mice. Circulation. 99:2157–2163. 1999.
Zhang GX., Kimura S., Nishiyama A., Shokoji T., Rahman M., Yao L., Nagai Y., Fujisawa Y., Miyatake A., Abe Y. Cardiac oxidative stress in acute and chronic isoproterenol-infused rats. Cardiovasc Res. 65:230–238. 2005.

Zhang J., Knapton A., Lipshultz SE., Weaver JL., Herman EH. Isoproterenol-induced cardiotoxicity in sprague-dawley rats: correlation of reversible and irreversible myocardial injury with release of cardiac troponin T and roles of iNOS in myocardial injury. Toxicol Pathol. 36:277–278. 2008.

Zhang P., Xu X., Hu X., van Deel ED., Zhu G., Chen Y. Inducible nitric oxide synthase deficiency protects the heart from systolic overload-induced ventricular hypertrophy and congestive heart failure. Circ Res. 100:1089–1098. 2007.

Zingarelli B., Hake PW., Yang Z., O'Connor M., Denenberg A., Wong HR. Absence of inducible nitric oxide synthase modulates early reperfusion-induced NF-kappaB and AP-1 activation and enhances myocardial damage. Faseb J. 16:327–342. 2002.
Go to : 
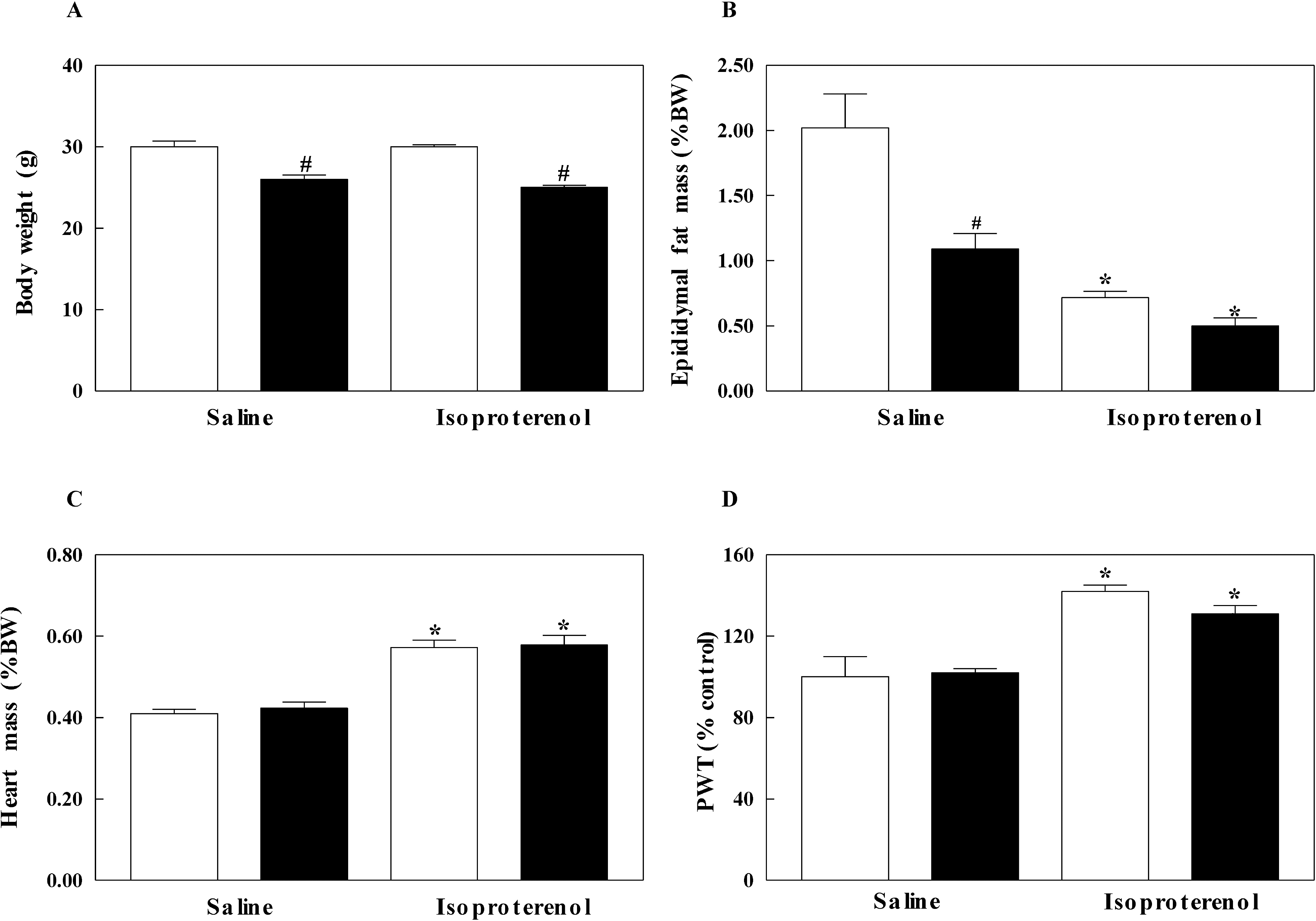 | Fig. 1.Body weight (A), epididymal fat mass (B), heart weight (C) and posterior wall thickness (PWT) of left ventricle (D) in inducible nitric oxide synthase (iNOS) knockout (black bar) and wild-type (white bar) mice infused with saline or isoproterenol. The experimental cases in each group are 6 to 9. The results are presented as mean±SE. ∗p<0.05 vs. saline-infused corresponding control in wild-type and iNOS knockout and #p<0.05 vs. corresponding wild-type in saline and isoproterenol groups. |
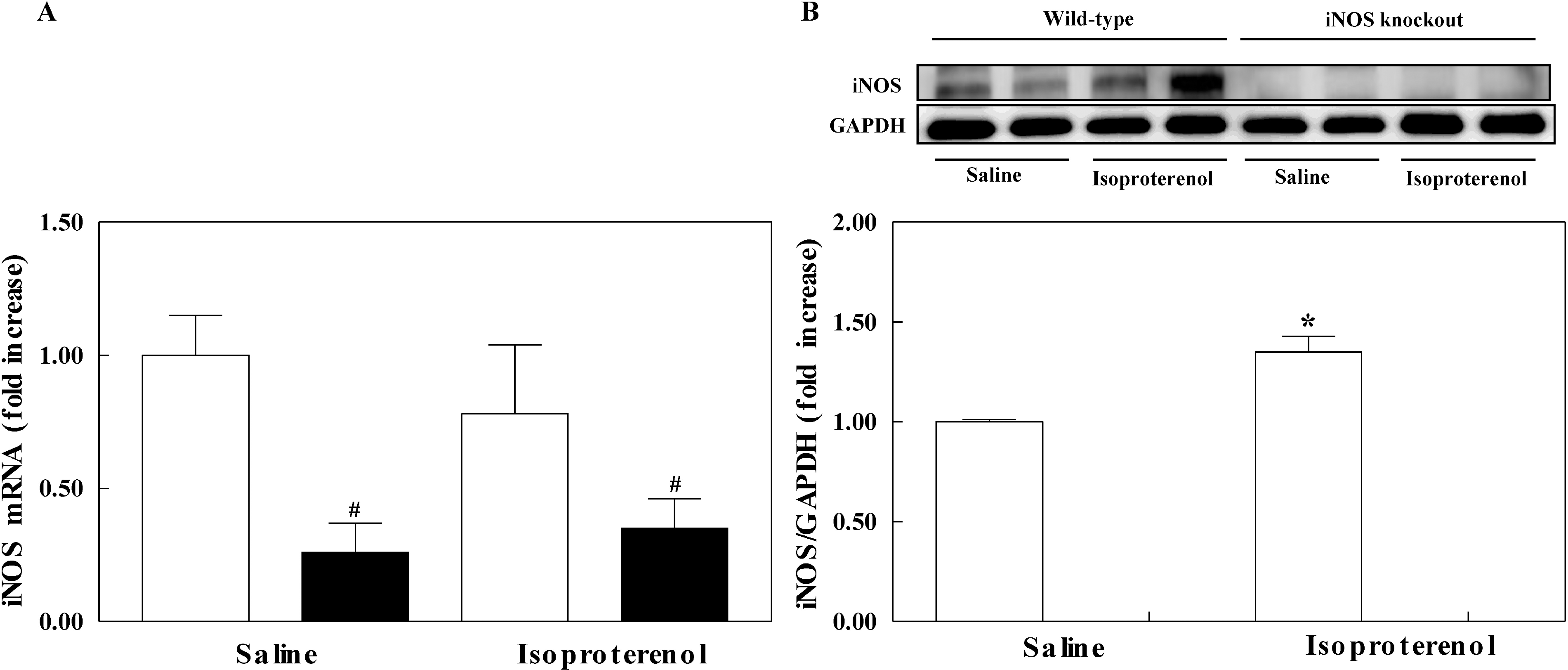 | Fig. 2.The effect of isoporterenol infusion on the mRNA expression (A) and protein level (B) of left ventricle in inducible nitric oxide synthase (iNOS) knockout (black bar) and wild-type mice (white bar). The experimental cases in each group are 4 to 6. The results are presented as mean±SE. ∗p<0.05 vs. saline-infused corresponding control in wild-type and iNOS knockout and #p<0.05 vs. corresponding wild-type in saline and isoproterenol groups. |
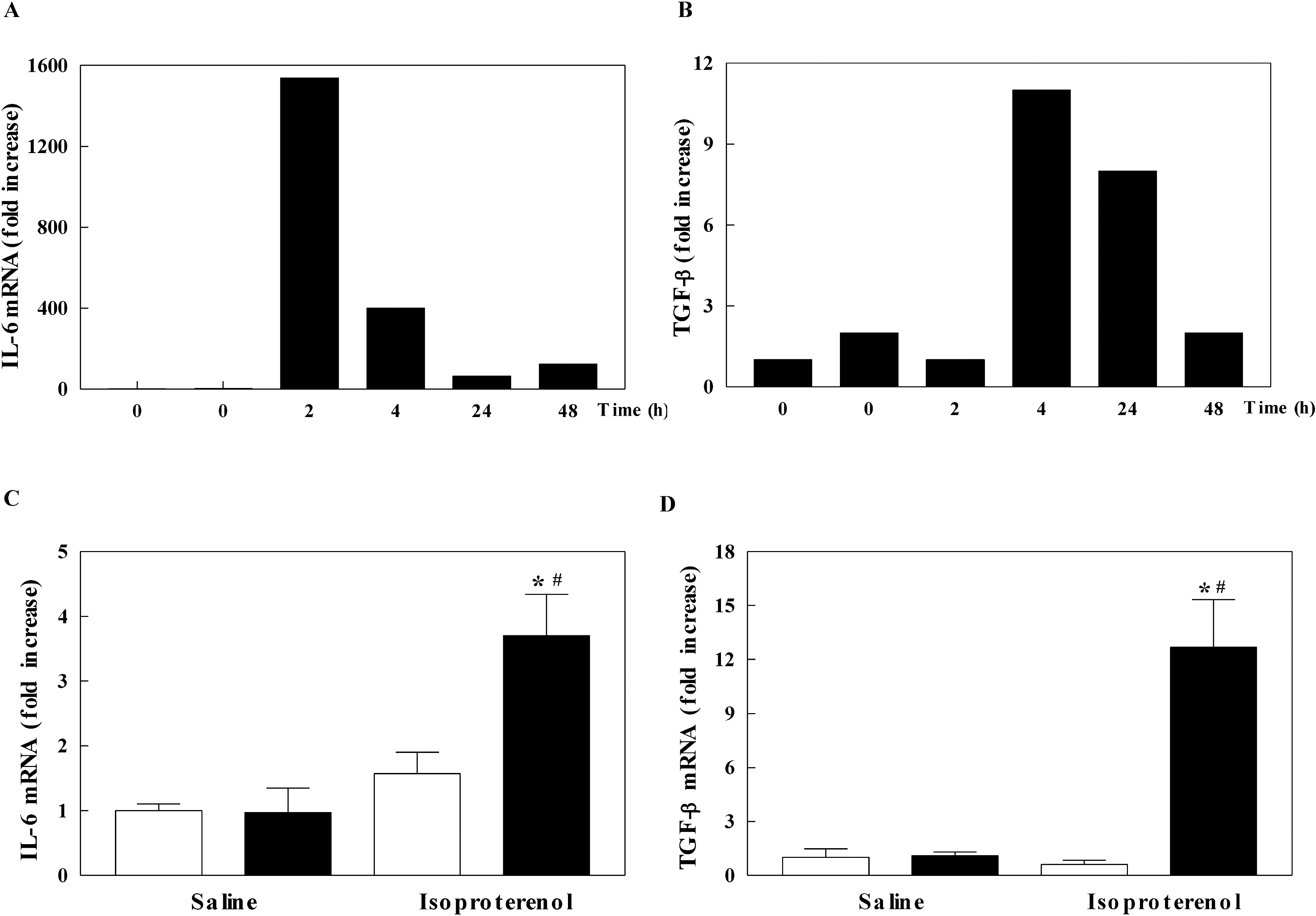 | Fig. 3.The mRNA levels of interleukin-6 (IL-6) and transforming growth factor-β (TGF-β) in the left ventricle of mice. The mRNA level of IL-6 (A) and TGF-β (B) in isoproterenol-infused wild-type mice in a time dependent manner. The mRNA level of IL-6 (C) and TGF-β (D) in inducible nitric oxide synthase (iNOS) knockout (black bar) and wild-type (white bar) mice infused with saline or isoproterenol. The experimental cases in C and D are 6 to 9 in each group. The results are presented as mean±SE. ∗p<0.05 vs. saline-infused iNOS knockout and #p<0.05 vs. isoproterenol-infused wild-type. |
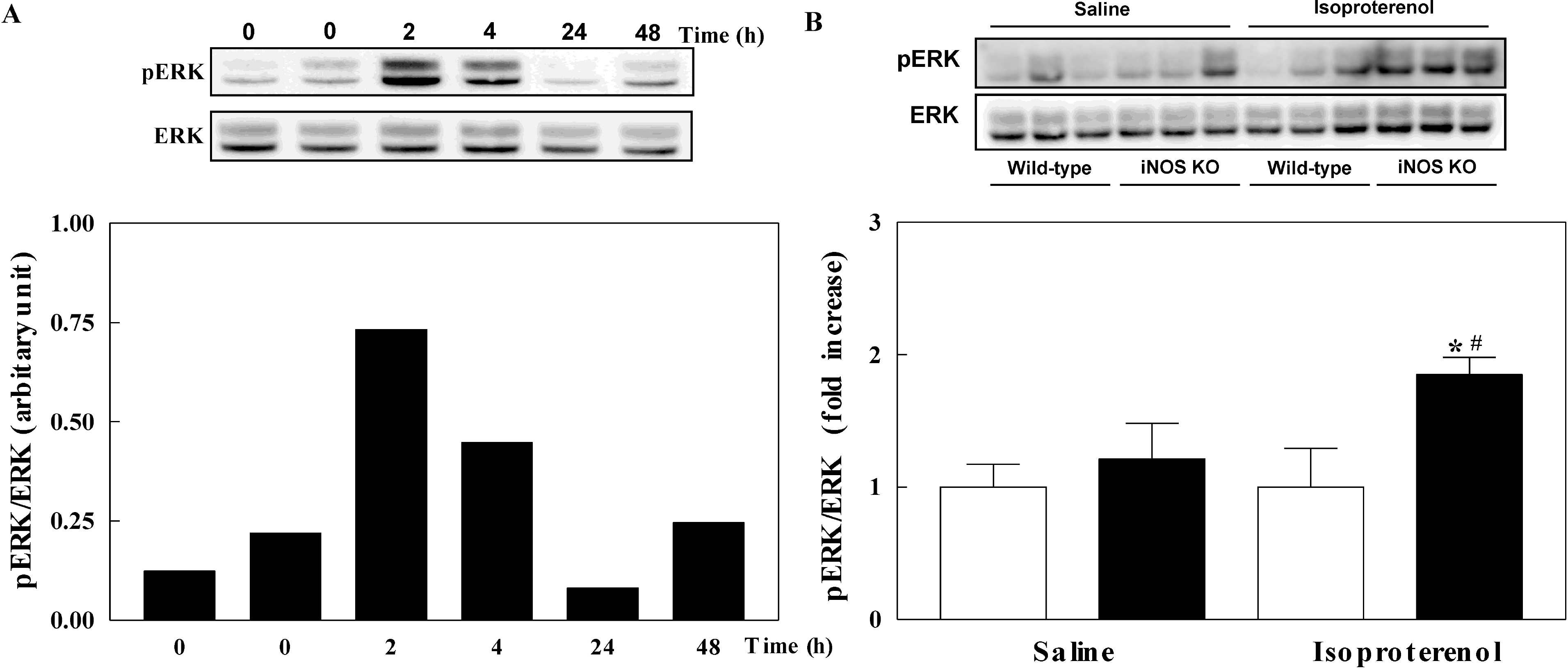 | Fig. 4.Phosphorylation of extracellular signal-regulated kinases (ERK) in the left ventricle of mice. Phosphorylation of ERK in isoproterenol-infused wild-type mice in a time dependent manner (A). Phosphorylation of ERK in inducible nitric oxide synthase (iNOS) knockout (black bar) and wild-type (white bar) mice infused with saline or isoproterenol (B). The experimental cases in B are 6 to 9 in each group. The results are presented as mean±SE. ∗p<0.05 vs. saline-infused iNOS knockout and #p<0.05 vs. isoproterenol-infused wild-type. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download