Abstract
This study was undertaken to elucidate the underlying mechanisms of ATP depletion-induced membrane transport dysfunction and cell death in renal proximal tubular cells. ATP depletion was induced by incubating cells with 2.5 mM potassium cyanide (KCN)/0.1 mM iodoacetic acid (IAA), and membrane transport function and cell viability were evaluated by measuring Na+-dependent phosphate uptake and trypan blue exclusion, respectively. ATP depletion resulted in a decrease in Na+-dependent phosphate uptake and cell viability in a time-dependent manner. ATP depletion inhibited Na+-dependent phosphate uptake in cells, when treated with 2 mM ouabain, a Na+ pump-specific inhibitor, suggesting that ATP depletion impairs membrane transport functional integrity. Alterations in Na+-dependent phosphate uptake and cell viability induced by ATP depletion were prevented by the hydrogen peroxide scavenger such as catalase and the hydroxyl radical scavengers (dimethylthiourea and thiourea), and amino acids (glycine and alanine). ATP depletion caused arachidonic acid release and increased mRNA levels of cytosolic phospholipase A2 (cPLA2). The ATP depletion-dependent arachidonic acid release was inhibited by cPLA2 specific inhibitor AACOCF3. ATP depletion-induced alterations in Na+-dependent phosphate uptake and cell viability were prevented by AACOCF3. Inhibition of Na+-dependent phosphate uptake by ATP depletion was prevented by antipain and leupetin, serine/cysteine protease inhibitors, whereas ATP depletion-induced cell death was not altered by these agents. These results indicate that ATP depletion-induced alterations in membrane transport function and cell viability are due to reactive oxygen species generation and cPLA2 activation in renal proximal tubular cells. In addition, the present data suggest that serine/cysteine proteases play an important role in membrane transport dysfunction, but not cell death, induced by ATP depletion.
Go to : 
REFERENCES
Bolli R., Cannon RO., Speir E., Goldstein RE., Epstein SE. Role of cellular proteinases in acute myocardial infarction. I. Proteolysis in nonischemic and ischemic rat myocardium and the effects of antipain, leupeptin, pepstatin and chymostatin administered in vivo. J Am Coll Cardiol. 2:671–680. 1983.

Borkan SC., Schwartz JH. Role of oxygen free radical species in in vitro models of proximal tubular ischemia. Am J Physiol. 257:F114–F125. 1989.

Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 72:248–254. 1976.

Bunnachak D., Almeida AR., Wetzels JF., Gengaro P., Nemenoff RA., Burke TJ., Schrier RW. Ca2+ uptake, fatty acid, and LDH release during proximal tubule hypoxia: effects of mepacrine and dibucaine. Am J Physiol. 266:F196–F201. 1994.
Chatterjee PK., Todorovic Z., Sivarajah A., Mota-Filipe H., Brown PA., Stewart KN., Mazzon E., Cuzzocrea S., Thiemermann C. Inhibitors of calpain activation (PD150606 and E-64) and renal ischemia-reperfusion injury. Biochem Pharmacol. 69:1121–1131. 2005.

Choi KH., Edelstein CL., Gengaro P., Schrier RW., Nemenoff RA. Hypoxia induces changes in phospholipase A2 in rat proximal tubules: evidence for multiple forms. Am J Physiol. 269:F846–F853. 1995.
Choi WR., Ko SH., Cho SI., Woo JS., Jung JS., Lee SH., Kim YK. Role of phospholipase A2 in hypoxia-induced renal cell injury. Korean J Physiol Pharmacol. 3:93–100. 1999.
Dagher PC. Modeling ischemia in vitro: selective depletion of adenine and guanine nucleotide pools. Am J Physiol Cell Physiol. 279:C1270–1277. 2000.

Denis I., Pointillart A., Lieberherr M. Effects of growth hormone and insulin-like growth factor-I on the proliferation and differentiation of cultured pig bone cells and rat calvaria cells. Growth Regul. 4:123–130. 1994.
Devarajan P. Update on mechanisms of ischemic acute kidney injury. J Am Soc Nephrol. 17:1503–1520. 2006.

Devarajan P., Mishra J., Supavekin S., Patterson LT., Steven Potter S. Gene expression in early ischemic renal injury: clues towards pathogenesis, biomarker discovery, and novel therapeutics. Mol Genet Metab. 80:365–376. 2003.

Edelstein CL., Ling H., Schrier RW. The nature of renal cell injury. Kidney Int. 51:1341–1351. 1997.

Frangie C., Zhang W., Perez J., Dubois YC., Haymann JP., Baud L. Extracellular calpains increase tubular epithelial cell mobility. Implications for kidney repair after ischemia. J Biol Chem. 281:26624–26632. 2006.
Gores GJ., Flarsheim CE., Dawson TL., Nieminen AL., Herman B., Lemasters JJ. Swelling, reductive stress, and cell death during chemical hypoxia in hepatocytes. Am J Physiol. 257:C347–354. 1989.

Gores GJ., Nieminen AL., Fleishman KE., Dawson TL., Herman B., Lemasters JJ. Extracellular acidosis delays onset of cell death in ATP-depleted hepatocytes. Am J Physiol. 255:C315–322. 1988.

Gores GJ., Nieminen AL., Wray BE., Herman B., Lemasters JJ. Intracellular pH during “chemical hypoxia” in cultured rat hepatocytes. Protection by intracellular acidosis against the onset of cell death. J Clin Invest. 83:386–396. 1989.

Goto S., Nakamura H., Morooka H., Terao Y., Shibata O., Sumikawa K. Role of reactive oxygen in phospholipase A2 activation by ischemia/reperfusion of the rat kidney. J Anesth. 13:90–93. 1999.
Habib MM., Hodgson HJ., Davidson BR. The role of glycine in hepatic ischemia-reperfusion injury. Curr Pharm Des. 12:2953–2967. 2006.

Hagar H., Ueda N., Shah SV. Role of reactive oxygen metabolites in DNA damage and cell death in chemical hypoxic injury to LLC-PK1 cells. Am J Physiol. 271:F209–F215. 1996.

Halliwell B., Gutteridge JMC., Cross CE. Free radicals, antioxidants, and human disease: Where are we now? J Lab Clin Med. 119:598–620. 1992.
Humes HD., Nguyen VD., Cieslinski DA., Messana JM. The role of free fatty acids in hypoxia-induced injury to renal proximal tubule cells. Am J Physiol. 256:F688–696. 1989.

Joannidis M., Gstraunthaler G., Pfaller W. Xanthine oxidase: evidence against a causative role in renal reperfusion injury. Am J Physiol. 258:F232–236. 1990.

Kim SY., Kim CH., Yoo HJ., Kim YK. Effects of radical scavengers and antioxidant on ischemic acute renal failure in rabbits. Renal Failure. 21:1–11. 1999.

Kim YK., Lee SK., Ha MS., Woo JS., Jung JS. Differential role of reactive oxygen species in chemical hypoxia-induced cell injury in opossum kidney cells and rabbit renal cortical slices. Exp Nephrol. 10:275–284. 2002.

Lash LH., Tokarz JJ., Chen Z., Pedrosi BM., Woods EB. ATP depletion by iodoacetate and cyanide in renal distal tubular cells. J Pharmacol Exp Ther. 276:194–205. 1996.
Lash LH., Tokarz JJ., Chen Z., Pedrosi BM., Woods EB. ATP depletion by iodoacetate and cyanide in renal distal tubular cells. J Pharmacol Exp Ther. 276:194–205. 1996.
Liu X., Harriman JF., Schnellmann RG. Cytoprotective properties of novel nonpeptide calpain inhibitors in renal cells. J Pharmacol Exp Ther. 302:88–94. 2002.

Liu X., Rainey JJ., Harriman JF., Schnellmann RG. Calpains mediate acute renal cell death: role of autolysis and translocation. Am J Physiol Renal Physiol. 281:F728–738. 2001.

Matthys E., Patel Y., Kreisberg J., Stewart JH., Venkatachalam M. Lipid alterations induced by renal ischemia: pathogenic factor in membrane damage. Kidney Int. 26:153–161. 1984.

Nakamura H., Nemenoff RA., Gronich JH., Bonventre JV. Subcellular characteristics of phospholipase A2 activity in the rat kidney. Enhanced cytosolic, mitochondrial, and microsomal phospholipase A2 enzymatic activity after renal ischemia and reperfusion. J Clin Invest. 87:1810–1818. 1991.
Nangaku M., Eckardt KU. Hypoxia and the HIF system in kidney disease. J Mol Med. 85:1325–1330. 2007.

Nicotera P., McConkey DJ., Dypbukt JM., Jones DP., Orrenius S. Ca2+-activated mechanisms in cell killing. Drug Metab Rev. 20:193–201. 1989.
Nieminen AL., Gores GJ., Bond JM., Imberti R., Herman B., Lemasters JJ. A novel cytotoxicity screening assay using a multiwell fluorescence scanner. Toxicol Appl Pharmacol. 115:147–155. 1992.

Nistico R., Piccirilli S., Cucchiaroni ML., Armogida M., Guatteo E., Giampa C., Fusco FR., Bernardi G., Nistico G., Mercuri NB. Neuroprotective effect of hydrogen peroxide on an in vitro model of brain ischaemia. Br J Pharmacol. 153:1022–1029. 2008.
Paller MS. The cell biology of reperfusion injury in the kidney. J Investi Med. 42:632–639. 1994.
Pan C., Bai X., Fan L., Ji Y., Li X., Chen Q. Cytoprotection by glycine against ATP-depletion-induced injury is mediated by glycine receptor in renal cells. Biochem J. 390:447–453. 2005.

Portilla D., Creer MH. Plasmalogen phospholipid hydrolysis during hypoxic injury of rabbit proximal tubules. Kidney Int. 47:1087–1094. 1995.

Portilla D., Mandel LJ., Bar-Sagi D., Millington DS. Anoxia induces phospholipase A2 activation in rabbit renal proximal tubules. Am J Physiol. 262:F354–360. 1992.
Portilla D., Shah SV., Lehman PA., Creer MH. Role of cytosolic calcium-independent plasmalogen-selective phospholipase A2 in hypoxic injury to rabbit proximal tubules. J Clin Invest. 93:1609–1615. 1994.
Schnellmann RG., Yang X., Carrick JB. Arachidonic acid release in renal proximal tubule cell injuries and death. J Biochem Toxicol. 9:211–217. 1994.

Seyfried DM., Veyna R., Han Y., Li K., Tang N., Betts RL. Weinsheimer S, Chopp M, Anagli J. A selective cysteine protease inhibitor is non-toxic and cerebroprotective in rats undergoing transient middle cerebral artery ischemia. Brain Res. 901:94–101. 2001.
Street IP., Lin HK., Laliberte F., Ghomashchi F., Wang Z., Perrier H., Tremblay NM., Huang Z., Weech PK., Gelb MH. Slow- and tight-binding inhibitors of the 85-kDa human phospholipase A2. Biochemistry. 32:5935–5940. 1993.
Wang H., Harrison SD., Lemasters JJ., Herman B. Contribution of pH-dependent group II phospholipase A2 to chemical hypoxic injury in rat hepatocytes. FASEB J. 10:1319–1325. 1996.
Watabe M., Nakaki T. ATP depletion does not account for apoptosis induced by inhibition of mitochondrial electron transport chain in human dopaminergic cells. Neuropharmacology. 52:536–541. 2007.

Weinberg JM., Davis JA., Abarzua M., Rajan T. Cytoprotective effects of glycine and glutathione against hypoxic injury to renal tubules. J Clin Invest. 80:1446–1454. 1987.

Wetzels JF., Wang X., Gengaro PE., Nemenoff RA., Burke TJ., Schrier RW. Glycine protection against hypoxic but not phospholipase A2-induced injury in rat proximal tubules. Am J Physiol. 264:F94–F99. 1993.
Wetzels JF., Yu L., Wang X., Kribben A., Burke TJ., Schrier RW. Calcium modulation and cell injury in isolated rat proximal tubules. J Pharmacol Exp Ther. 267:176–180. 1993.
Yang X., Schnellmann RG. Proteinases in renal cell death. J Toxicol Environ Health. 48:319–332. 1996.

Yin M., Zhong Z., Connor HD., Bunzendahl H., Finn WF., Rusyn I., Li X., Raleigh JA., Mason RP., Thurman RG. Protective effect of glycine on renal injury induced by ischemia-reperfusion in vivo. Am J Physiol Renal Physiol. 282:F417–423. 2002.
Zager RA., Burkhart KM., Conrad DS., Gmur DJ., Iwata M. Phospholipase A2-induced cytoprotection of proximal tubules: potential determinants and specificity for ATP depletion-mediated injury. J Am Soc Nephrol. 7:64–72. 1996.
Zager RA., Johnson AC., Lund S., Randolph-Habecker J. Toll-like receptor (TLR4) shedding and depletion: acute proximal tubular cell responses to hypoxic and toxic injury. Am J Physiol Renal Physiol. 292:F304–312. 2007.

Zager RA., Schimpf BA., Gmur DJ., Burke TJ. Phospholipase A2 activity can protect renal tubules from oxygen deprivation injury. Proc Natl Acad Sci USA. 90:8297–8301. 1993.
Go to : 
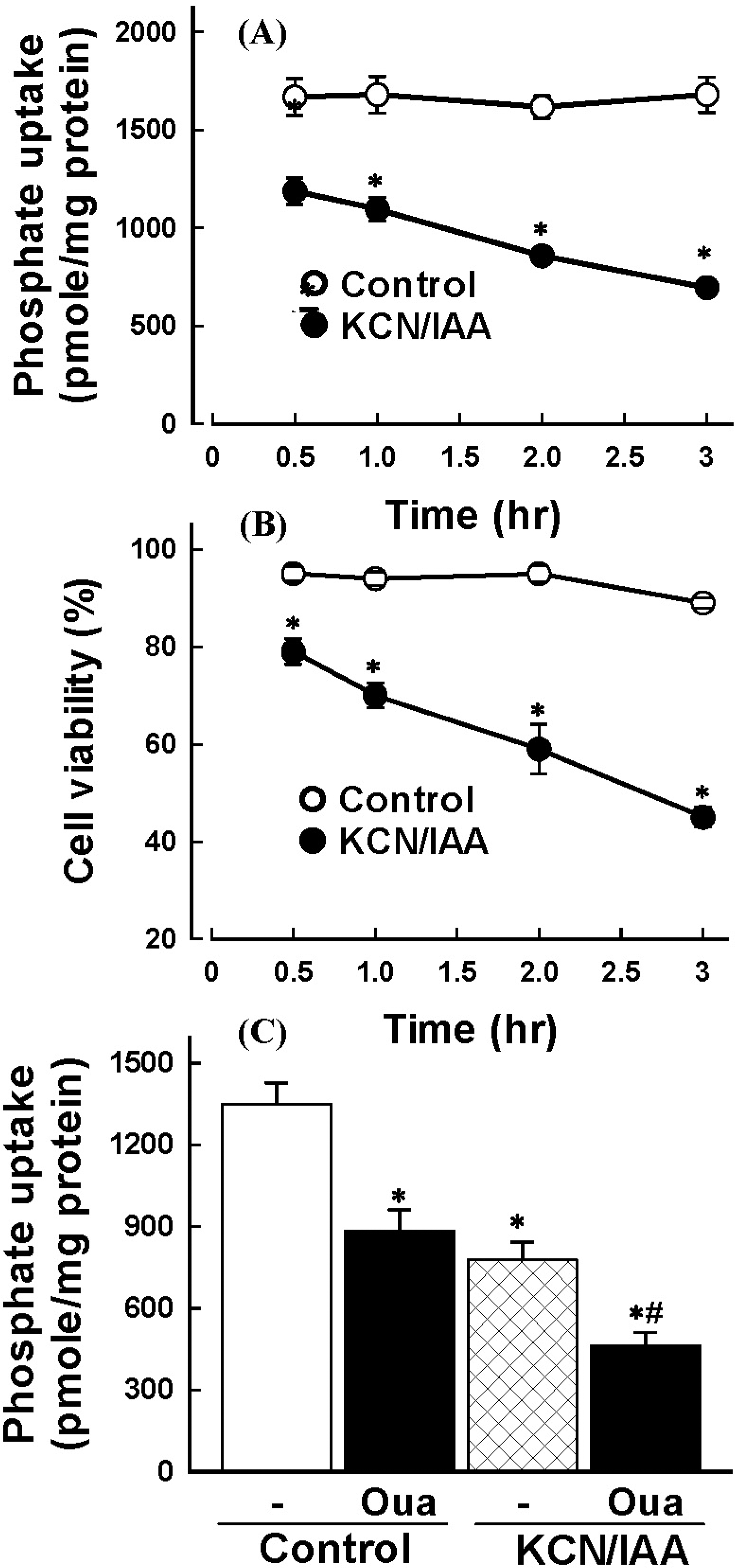 | Fig. 1.Effect of ATP depletion on Na+-dependent phosphate uptake (A) and cell viability (B) in primary cultured renal proximal tubular cells. Cells were exposed to medium with 2.5 mM potassium cyanide/0.1 mM iodoacetic acid (KCN/IAA) or without (Control) for 0~3 hr at 37°C. Na+-dependent phosphate uptake and cell viability were measured as described in ‘Materials and Methods'. Data are mean±SEM of four independent experiments performed in duplicate. ∗p<0.05 compared with control. (C) Effect of ATP depletion on Na+-dependent phosphate uptake in primary cultured renal proximal tubular cells which were treated with ouabain. Cells were exposed to medium with 2.5 mM potassium cyanide/0.1 mM iodoacetic acid (KCN/IAA) or without (Control) in the presence or absence of 2 mM ouabain for 120 min at 37°C. Na+-dependent phosphate uptake was measured as described in ‘Materials and Methods'. Data are mean±SEM of four independent experiments performed in duplicate. ∗p<0.05 compared with control; #p<0.05 compared with ouabain alone. |
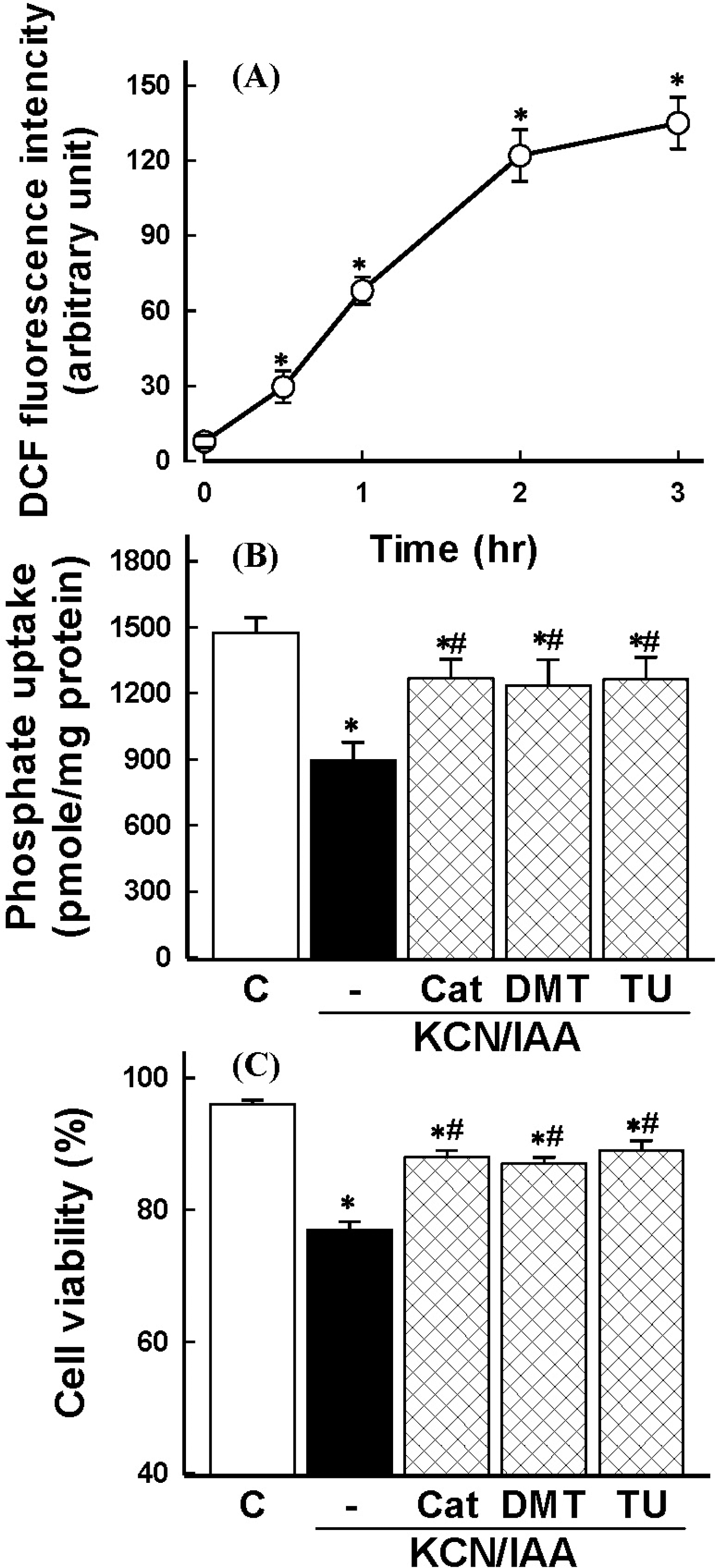 | Fig. 2.(A) Time course of reactive oxygen species (ROS) generation in primary cultured renal proximal tubular cells during exposure to ATP depletion. Cells were exposed to 2.5 mM potassium cyanide/0.1 mM iodoacetic acid (KCN/IAA) for various times and ROS generation was measured. Shown is the net increase of DCF fluorescence (arbitrary units) calculated by subtracting the values for the control cells from the corresponding values for KCN/IAA-treated cells. Data are mean±SEM of three independent experiments performed in duplicate. ∗p<0.05 compared with zero (0) time. (B-C) Effect of radical scavengers on inhibition of phosphate uptake (B) and cell death (C) induced by ATP depletion. Cells were exposed to KCN/IAA in the presence or absence of 500 units/ml catalase (Cat), 30 mM dimethylthiourea (DMTU), or 30 mM thiourea (TU) for 3 hr at 37°C. Na+-dependent phosphate uptake and cell viability were measured as described in ‘Materials and Methods'. Data are mean±SEM of four independent experiments performed in duplicate. ∗p<0.05 compared with control; #p<0.05 compared with KCN/IAA alone. |
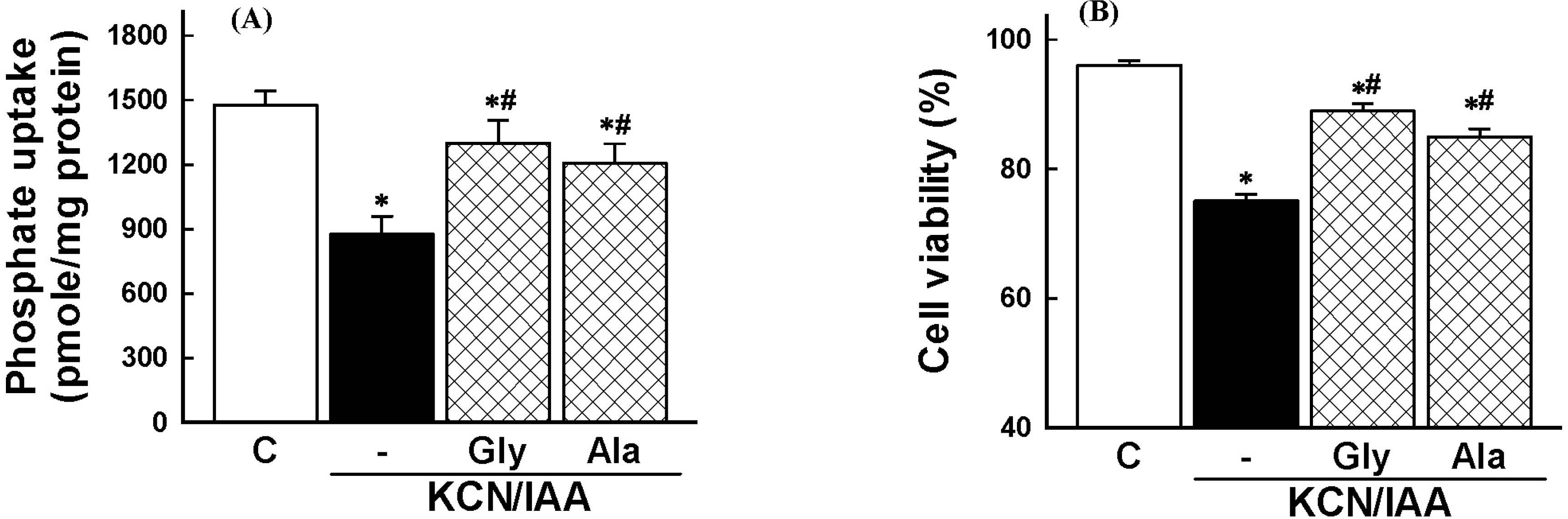 | Fig. 3.Effects of amino acids on inhibition of phosphate uptake (A) and cell death induced by ATP depletion, (B) in primary cultured renal proximal tubular cells. Cells were exposed to 2.5 mM potassium cyanide/0.1 mM iodoacetic acid (KCN/IAA) in the presence or absence of 5 mM glycine (Gly) or 5 mM alanine (Ala) for 3 hr at 37°C. Na+-dependent phosphate uptake and cell viability were measured as described in ‘Materials and Methods'. Data are mean±SEM of four independent experiments performed in duplicate. ∗p<0.05 compared with control; #p<0.05 compared with KCN/IAA alone. |
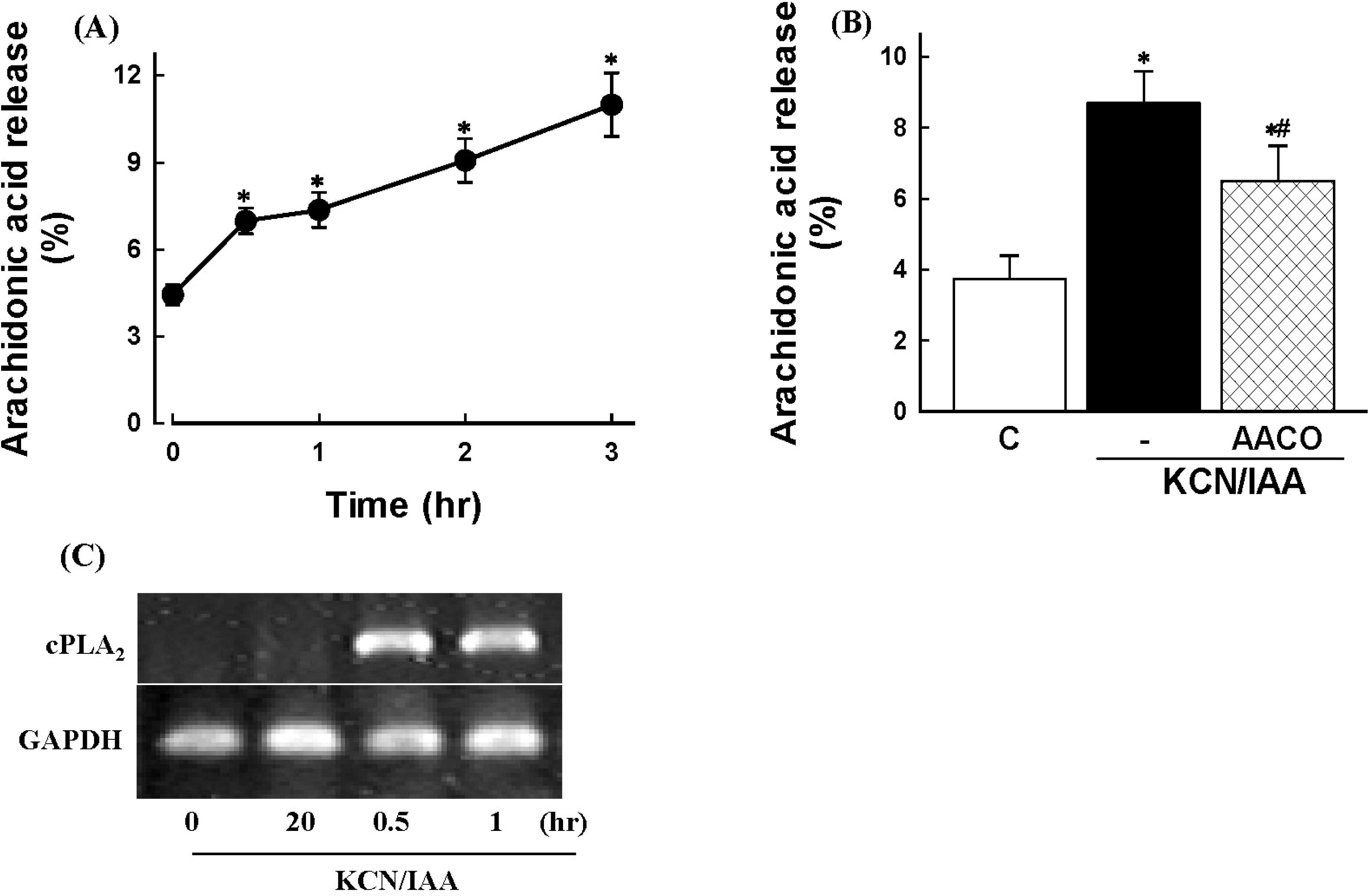 | Fig. 4.Effects of ATP depletion on arachidonic acid release in primary cultured renal proximal tubular cells. Cells were prelabeled with [3H]arachidonic acid for 20 hr, washed, and exposed to 2.5 mM potassium cyanide/0.1 mM iodoacetic acid (KCN/IAA) for various times (A) or for 3 hr in the presence or absence of 20 μM AACOCF3 (B). Data are mean±SEM of four independent experiments performed in duplicate. ∗p<0.05 compared with zero time (A) and control (B); #p<0.05 compared with KCN/IAA alone. (C) RT-PCR analysis of cPLA2 mRNA in cells subjected to ATP depletion. Cells were exposed to KCN/IAA for 0.2, 0.5, and 1 hr, and mRNA levels of were measured as described in ‘Materials and Methods'. |
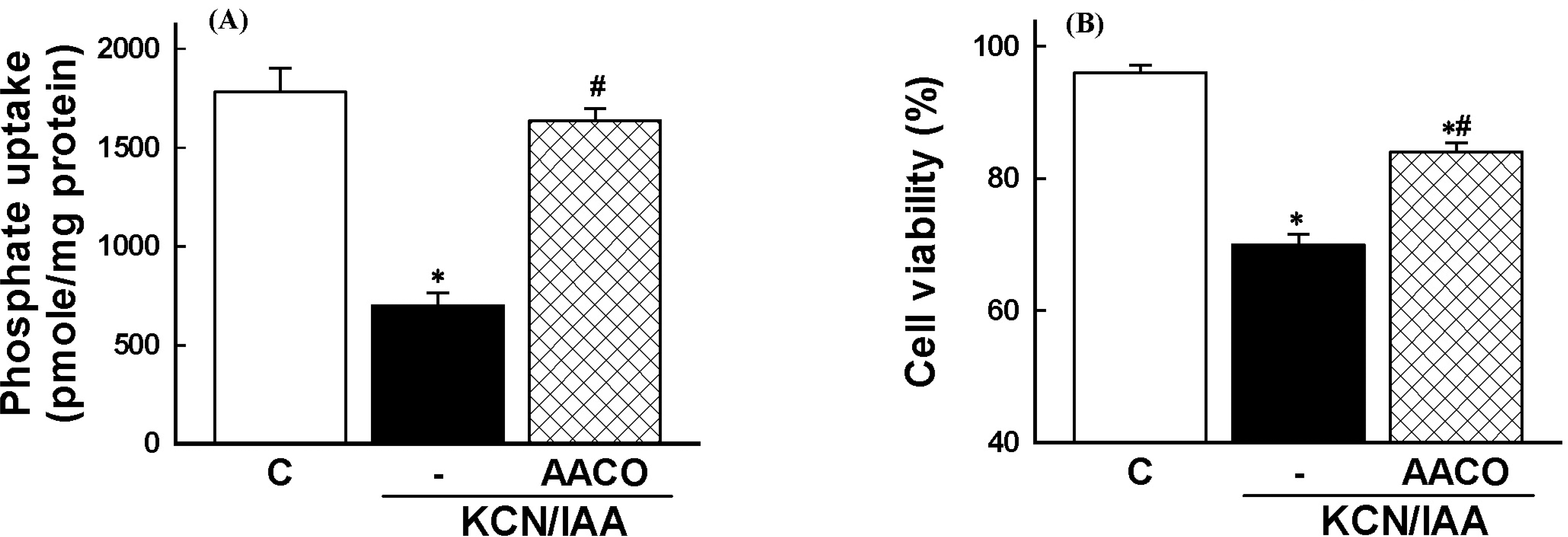 | Fig. 5.Effects of phospholipase A2 inhibitor on inhibition of Na+-dependent phosphate uptake (A) and cell death (B), induced by ATP depletion, in primary cultured renal proximal tubular cells. Cells were exposed to 2.5 mM potassium cyanide/0.1 mM iodoacetic acid (KCN/IAA) in the presence or absence of 20 μM AACOCF3 for 3 hr at 37°C. Na+-dependent phosphate uptake and cell viability were measured as described in ‘Materials and Methods'. Data are mean±SEM of four independent experiments performed in duplicate. ∗p<0.05 compared with control; #p<0.05 compared with KCN/IAA alone. |
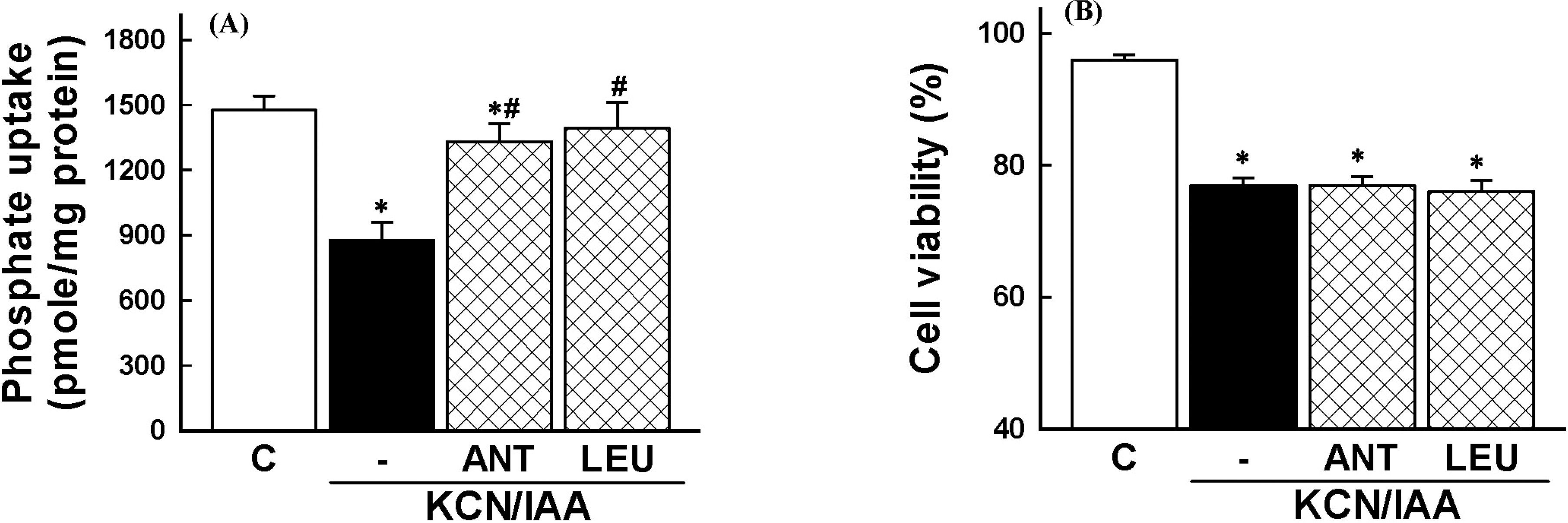 | Fig. 6.Effects of serine/cysteine protease inhibitors on inhibition of phosphate uptake (A) and cell death (B), induced by ATP depletion, in primary cultured renal proximal tubular cells. Cells were exposed to 2.5 mM potassium cyanide/0.1 mM iodoacetic acid (KCN/IAA) in the presence or absence of 50 μM antipain (ANT) or 100 μM leupetin (LEU) for 3 hr at 37°C. Na -dependent phosphate uptake and cell viability were measured as described in ‘Materials and Methods'. Data are mean±SEM of four independent experiments performed in duplicate. ∗p<0.05 compared with control; #p<0.05 compared with KCN/IAA alone. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download