Abstract
Aprotinin is used clinically in cardiopulmonary bypass surgery to reduce transfusion requirements and the inflammatory response. The mechanism of action for the anti-inflammatory effects of aprotinin is still unclear. We examined our hypothesis whether inhibitory effects of aprotinin on cytokine-induced inducible nitric oxide synthase (iNOS) expression (IL-1β plus TNF-α), reactive oxygen species (ROS) generation, and vascular smooth muscle cell (VSMC) proliferation were due to HO-1 induction in rat VSMCs. Aprotinin induced HO-1 protein expression in a dose-dependent manner, which was potentiated during inflammatory condition. Aprotinin reduced cytokine mixture (CM)-induced iNOS expression in a dose dependent manner. Furthermore, aprotinin reduced CM-induced ROS generation, cell prolife-ration, and phosphorylation of JNK but not of P38 and ERK 1/2 kinases. Aprotinin effects were reversed by pre-treatment with the HO-1 inhibitor, tin protoporphyrin IX (SnPPIX). HO-1 is therefore closely involved in inflammatory-stimulated VSMC proliferation through the regulation of ROS generation and JNK phosphorylation. Our results suggest a new molecular basis for aprotinin anti-inflammatory properties.
REFERENCES
Asimakopoulos G., Thompson R., Nourshargh S., Lidington EA., Mason JC., Ratnatunga CP., Haskard DO., Taylor KM., Landis RC. An anti-inflammatory property of aprotinin detected at the level of leukocyte extravasation. J Thorac Cardiovasc Surg. 120:361–369. 2000.

Brister SJ., Ofosu FA., Buchanan MR. Thrombin generation during cardiac surgery: is heparin the ideal anticoagulant? Thromb Haemost. 70:259–262. 1993.

Clark JE., Foresti R., Green CJ., Motterlini R. Dynamics of haem oxygenase-1 expression and bilirubin production in cellular protection against oxidative stress. Biochem J. 348:615–619. 2000.

Day JR., Taylor KM., Lidington EA., Mason JC., Haskard DO., Randi AM., Landis RC. Aprotinin inhibits proinflammatory activation of endothelial cells by thrombin through the protease-activated receptor 1. J Thorac Cardiovasc Surg. 131:21–27. 2006.

Duckers HJ., Boehm M., True AL., Yet SF., San H., Park JL., Clinton Webb R., Lee ME., Nabel GJ., Nabel EG. Heme oxygenase-1 protects against vascular constriction and proliferation. Nat Med. 7:693–698. 2001.

Durante W., Kroll MH., Christodoulides N., Peyton KJ., Schafer AI. Nitric oxide induces heme oxygenase-1 gene expression and carbon monoxide production in vascular smooth muscle cells. Circ Res. 80:557–64. 1997.

Hill GE., Springall DR., Robbins RA. Aprotinin is associated with a decrease in nitric oxide production during cardiopulmonary bypass. Surgery. 121:449–455. 1997.

Guikema BJ., Ginnan R., Singer HA., Jourd'heuil D. Catalase potentiates interleukin-1beta-induced expression of nitric oxide synthase in rat vascular smooth muscle cells. Free Radic Biol Med. 38:597–605. 2005.
Johnson RA., Lavesa M., Askari B., Abraham NG., Nasjletti A. A heme oxygenase product, presumably carbon monoxide, mediates a vasodepressor function in rats. Hypertension. 25:166–169. 1995.

Juan SH., Cheng TH., Lin HC., Chu YL., Lee WS. Mechanism of concentration-dependent induction of heme oxygenase-1 by resveratrol in human aortic smooth muscle cells. Biochem Pharmacol. 69:41–48. 2005.

Kapturczak MH., Wasserfall C., Brusko T., Campbell-Thompson M., Ellis TM., Atkinson MA., Agarwal A. Heme oxygenase-1 modulates early inflammatory responses: evidence from the heme oxygenase-1-deficient mouse. Am J Pathol. 165:1045–1053. 2004.
Keyse SM., Tyrrell RM. Heme oxygenase is the major 32-kDa stress protein induced in human skin fibroblasts by UVA radiation, hydrogen peroxide, and sodium arsenite. Proc Natl Acad Sci USA. 86:99–103. 1989.

Landis RC., Asimakopoulos G., Poullis M., Haskard DO., Taylor KM. The antithrombotic and antiinflammatory mechanisms of action of aprotinin. Ann Thorac Surg. 72:2169–2175. 2001.

Landis RC., Haskard DO., Taylor KM. New antiinflammatory and platelet-preserving effects of aprotinin. Ann Thorac Surg. 72:S1808–1813. 2001.

Lee TS., Chang CC., Zhu Y., Shyy JY. Simvastatin induces heme oxygenase-1: a novel mechanism of vessel protection. Circulation. 110:1296–1302. 2004.
Lee TS., Chau LY. Heme oxygenase-1 mediates the anti-inflammatory effect of interleukin-10 in mice. Nat Med. 8:240–246. 2002.

Lee TS., Tsai HL., Chau LY. Induction of heme oxygenase-1 expression in murine macrophages is essential for the anti-inflammatory effect of low dose 15-deoxy-Δ12,14-prostaglandin J2. J Biol Chem. 278:19325–19330. 2003.
Maines MD. Heme oxygenase: function, multiplicity, regulatory mechanisms, and clinical applications. FASEB J. 2:2557–2568. 1988.

McCoubrey WK Jr., Huang TJ., Maines MD. Isolation and characterization of a cDNA from the rat brain that encodes hemoprotein heme oxygenase-3. Eur J Bioche. 247:725–732. 1997.

Minamino T., Christou H., Hsieh CM., Liu Y., Dhawan V., Abraham NG., Perrella MA., Mitsialis SA., Kourembanas S. Targeted expression of heme oxygenase-1 prevents the pulmonary inflammatory and vascular responses to hypoxia. Proc Natl Acad Sci USA. 98:8798–8803. 2001.

Motterlini R., Foresti R., Intaglietta M., Winslow RM. NO-mediated activation of heme oxygenase: endogenous cytoprotection against oxidative stress to endothelium. Am J Physio. 270:H107–H114. 1996.

Otterbein LE., Zuckerbraun BS., Haga M., Liu F., Song R., Usheva A., Stachulak C., Bodyak N., Smith RN., Csizmadia E., Tyagi S., Akamatsu Y., Flavell RJ., Billiar TR., Tzeng E., Bach FH., Choi AM., Soares MP. Carbon monoxide suppresses arteriosclerotic lesions associated with chronic graft rejection and with balloon injury. Nat Med. 9:183–190. 2003.

Poss KD., Tonegawa S. Reduced stress defense in heme oxygenase deficient cells. Proc Natl Acad Sci USA. 94:10925–10930. 1997.
Poullis M., Manning R., Laffan M., Haskard DO., Taylor KM., Landis RC. The antithrombotic effect of aprotinin: actions mediated via the protease activated receptor 1. J Thorac Cardiovasc Surg. 120:370–378. 2000.
Tenhunen R., MArver HS., Schmid R. The enzymatic conversion of heme to bilirubin by microsomal heme owygenase. Proc Natl Acad Sci USA. 61:748–755. 1968.
Wachtfogel YT., Kucich U., Hack CE., Gluszko P., Niewiarowski S., Colman RW., Edmunds LH Jr. Aprotinin inhibits the contact, neutrophil, and platelet activation systems during simulated extracorporeal perfusion. J Thorac Cardiovasc Surg. 106:1–9. 1993.

Wagner CT., Durante W., Christodoulides N., Hellums JD., Schafer AI. Hemodynamic forces induce the expression of heme oxygenase in cultured vascular smooth muscle cells. J Clin Invest. 100:589–596. 1997.

Yachie A., Niida Y., Wada T., Igarashi N., Kaneda H. Oxidative stress causes enhanced endothelial cell injury in human heme oxygenase-1 deficiency. J Clin Invest. 103:129–135. 1999.

Yoshinaga T., Sassa S., Kappas A. Purification and properties of bovine spleen heme oxygenase: amino acid composition and sites of action of inhibitors of heme oxygenase. J Biol Chem. 257:7778–7785. 1982.
Fig. 1.
Aprotinin induces HO-1 on cytokine-stimulated VSMCs. VSMCs were pre-treated with aprotinin (1~100 μM) for 1 hr and treated with cytokine (10 ng/ml IL-1β plus 25 ng/ml TNF-α, I + T) for 12 hrs prior to measurement of HO-1 proteins by Western blotting (A, C). Cells were pre-treated with aprotinin (10 μM) and SnPPIX (1 μM) for 1 hr, and were treated with cytokine for 12 hrs prior to measurement of HO-1 mRNA by RT-PCR (B, D). Results represent three independent experiments. ∗p value<0.001 compared with control, ∗∗p value<0.001 compared with I + T plus aprotinin.
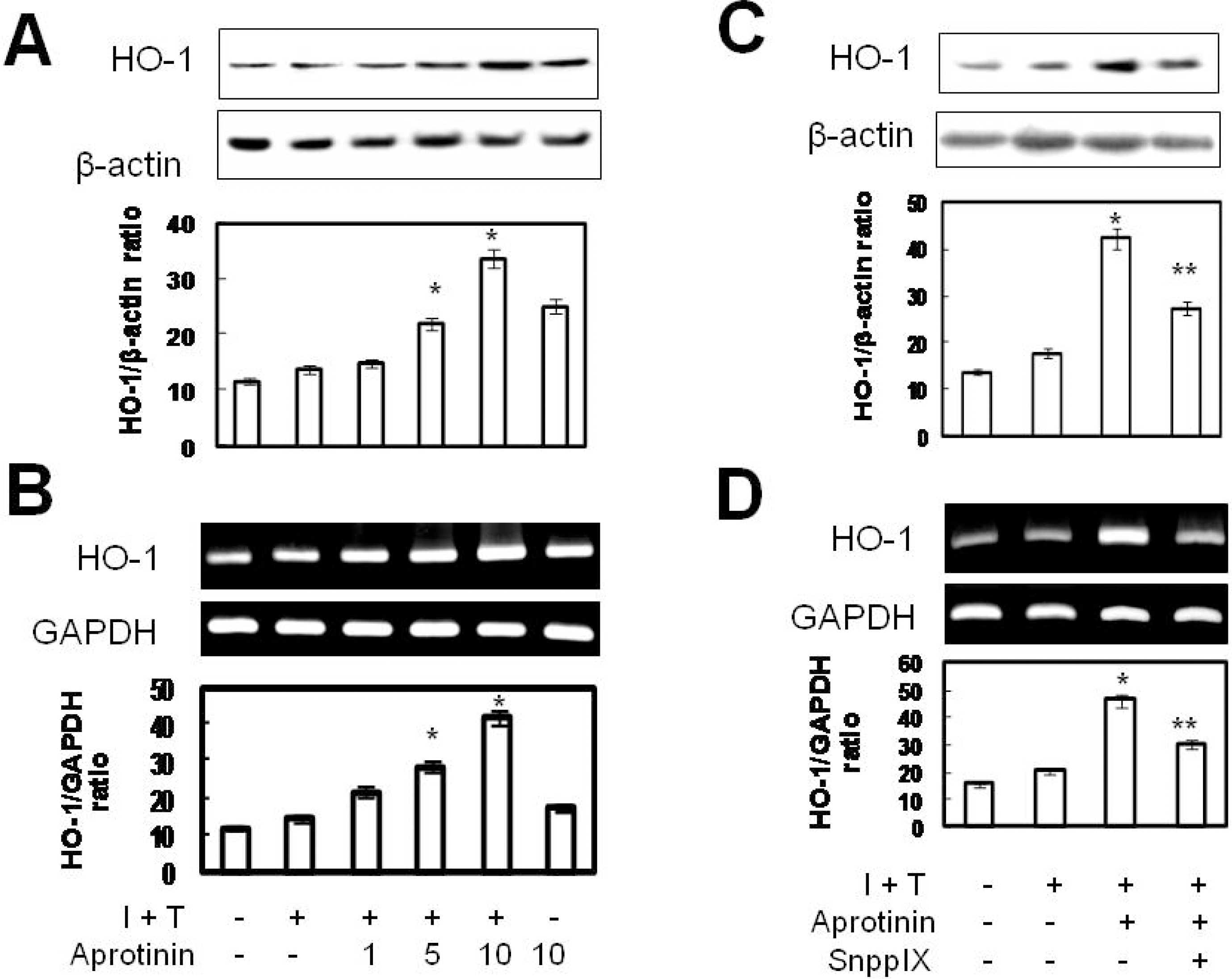
Fig. 2.
Aprotinin inhibits cytokine-induced iNOS expression in VSMCs through HO-1. VSMCs were pre-treated with aprotinin (1~100 μM), were treated with cytokine (I + T) for 12 hrs prior to measurement of iNOS protein expression (A), and for 24 hrs prior to measurement of nitrite production (B). Cells were pre-treated with aprotinin (10 μM) and SnPPIX (1 μM), and were treated with cytokine for 12 hrs prior to measurement of iNOS protein expression (C) and nitrite production (D). Data represent mean±SD values of four independent experiments. ∗p value<0.001 compared with control, ∗∗p value<0.001 compared with I + T, †p value <0.001 compared with aprotinin.
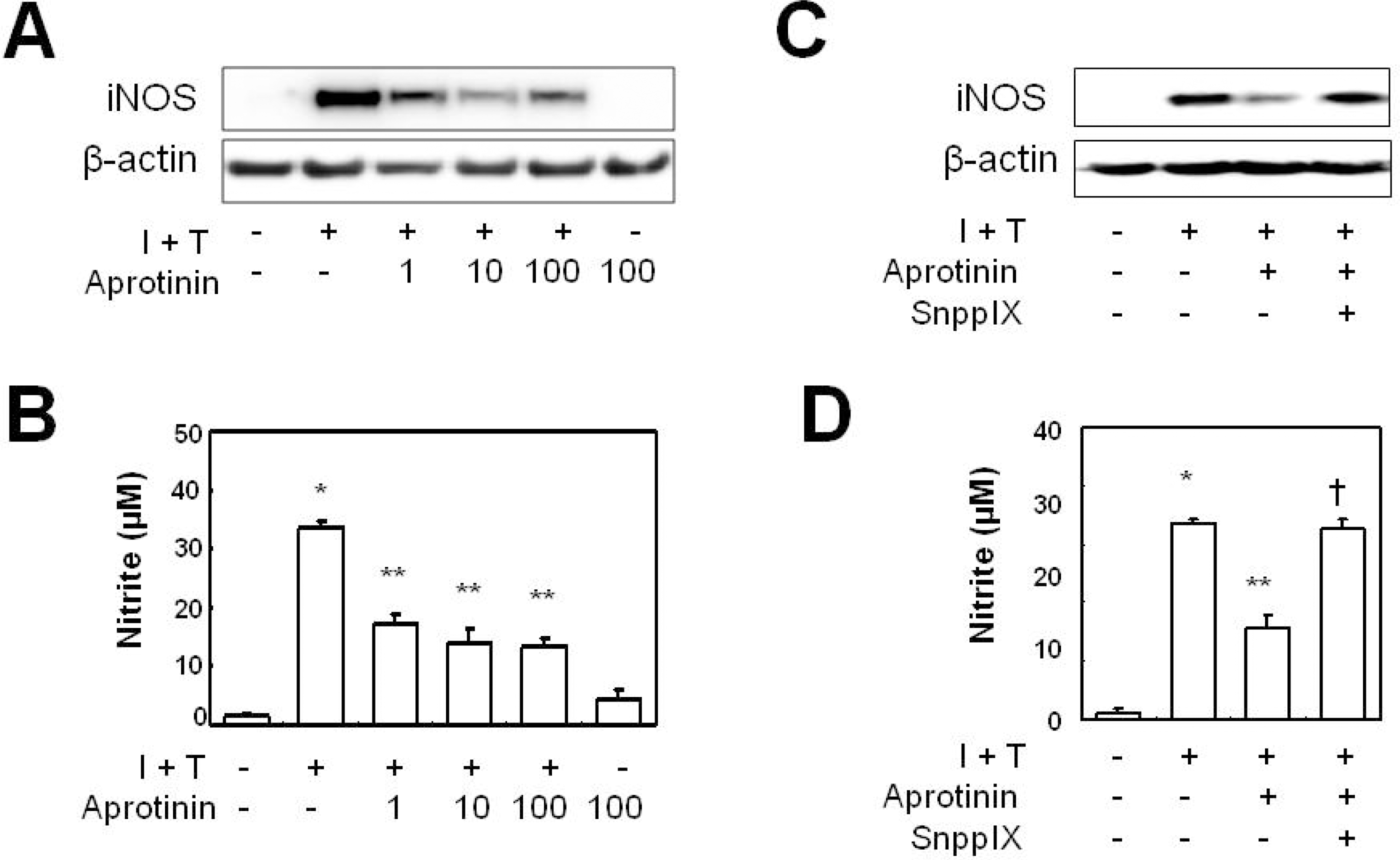
Fig. 3.
Aprotinin inhibits cytokine-induced VSMC proliferation through HO-1. VSMCs were pretreated with aprotinin (1~100 μM) for 1 hr, and were treated with cytokine (I+T) for 48 hrs prior to measurement of cell proliferation by the MTT assay (A) and for 4 days prior to counting cell numbers (B). Cells were pre-treated with aprotinin (10 μM) and SnPPIX (1 μM) for 1 hr, and were treated with cytokines for 48 hrs prior to measurement of cell proliferation by the MTT assay (C) and for 4 days prior to counting cell numbers (D). Data represent the mean±SD values of four independent experiments.
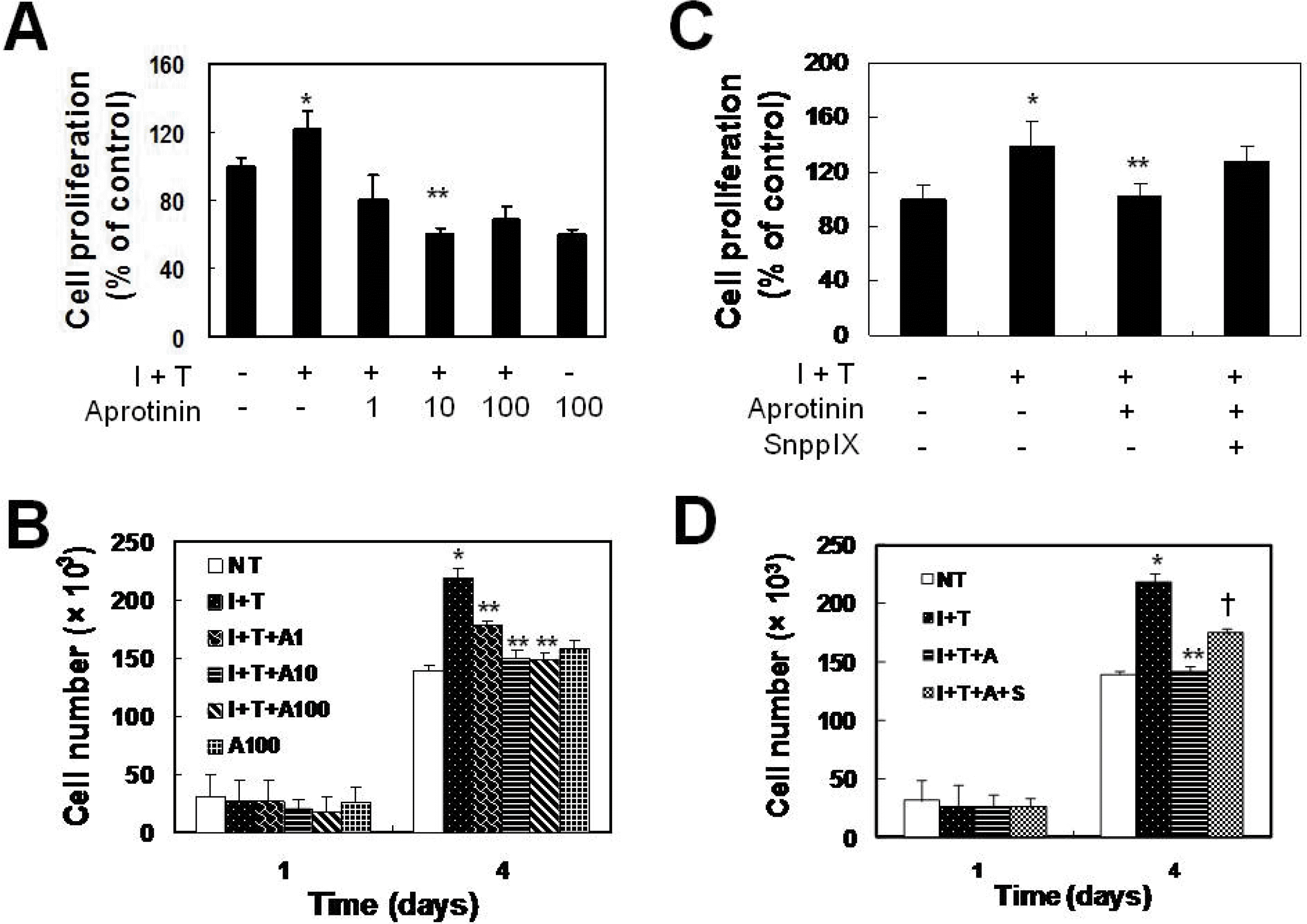
Fig. 4.
Aprotinin inhibits cytokine-induced ROS generation in VSMCs through HO-1. VSMCs were pre-treated with aprotinin (10 μM) and SnPPIX (1 μM) for 1 hr, and were treated with cytokine for 12 hrs prior to measurement of ROS generation by live image microscopy (A) and FACs analysis (B). ROS was detected using H2DCFHDA. Data represent the mean±SD values of four independent experiments.
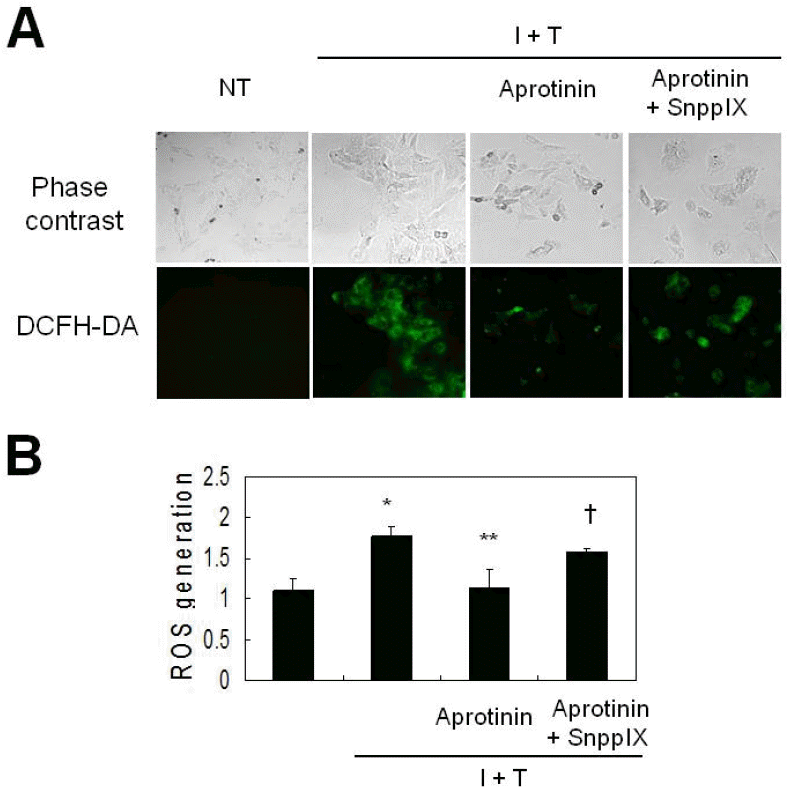
Fig. 5.
JNK plays a role in the prevention of cytokine-induced iNOS expression and proliferation. VSMCs were pre-treated with the MEK inhibitor (PD98059, 2.5 μM), p38 inhibitor (SB203580, 10 μM), and JNK inhibitor (10 μM) for 1 hr, and were treated with cytokines for 12 hrs prior to measurement of iNOS protein expression (A), for 24 hrs prior to measurement of nitrite production (B), for 48 hrs prior to measurement of cell proliferation by MTT assay (C), and for 4 days prior to measurement of cell proliferation by cell counts (D). Data represent the mean±SD values of four independent experiments. ∗p value<0.0001 compared with control, ∗∗p value < 0.0001 compared with I + T.
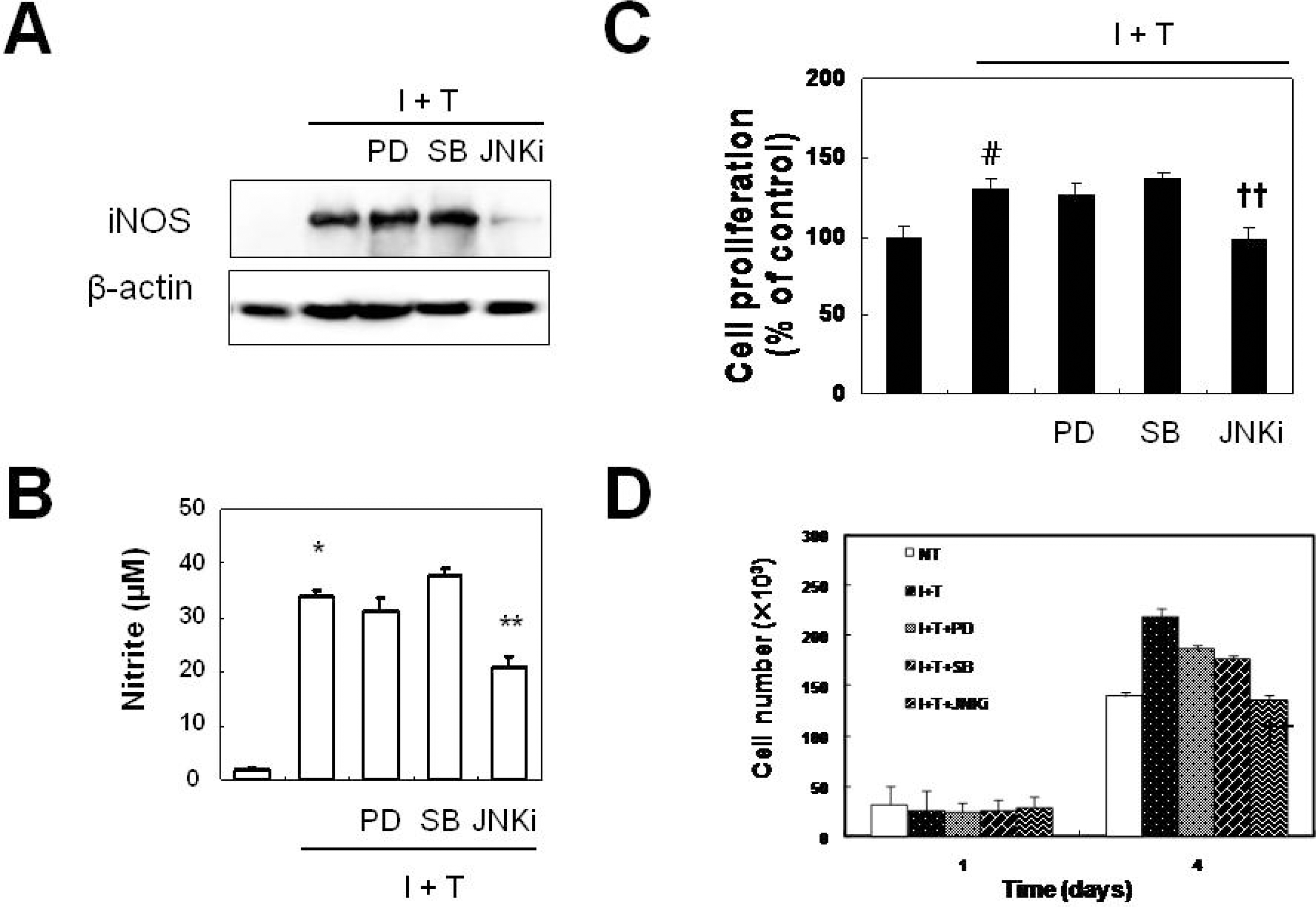
Fig. 6.
JNK plays a role in the prevention of cytokine-induced ROS generation in VSMCs. VSMCs were pre-treated with MEK inhibitor (PD98059, 2.5 μM), p38 inhibitor (SB203580, 10 μM), and JNK inhibitor (10 μM) for 1 hr, and were treated with cytokine for 12 hrs prior to measurement of ROS generation by live image microscopy (A) and by FACs analysis (B). Data represent the mean±SD values of four independent experiments.
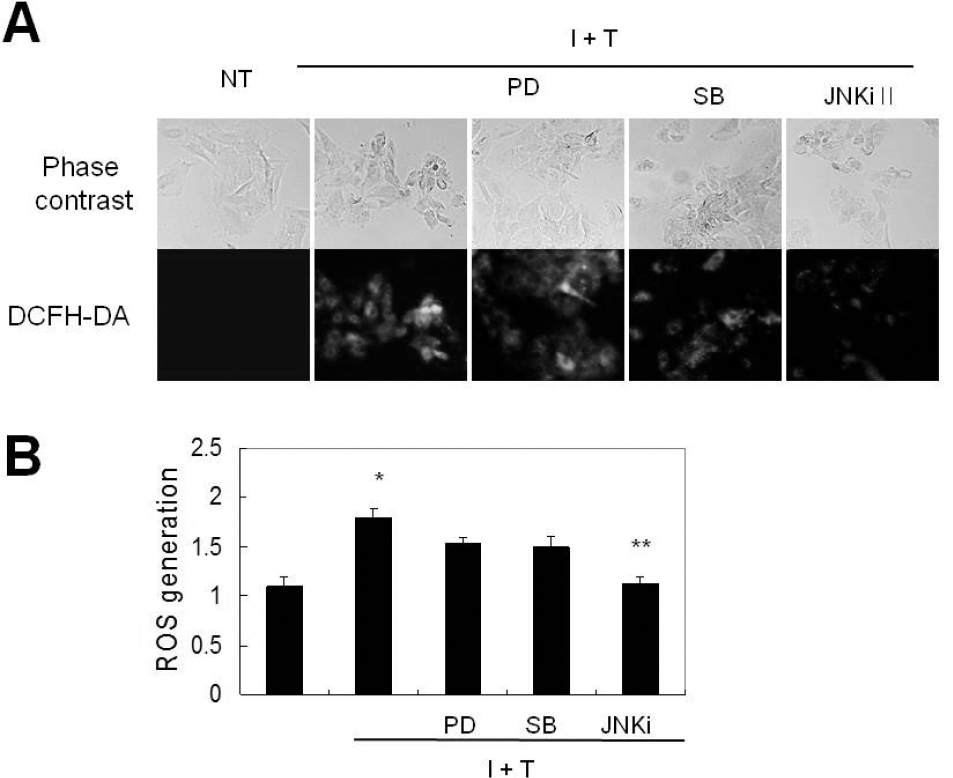
Fig. 7.
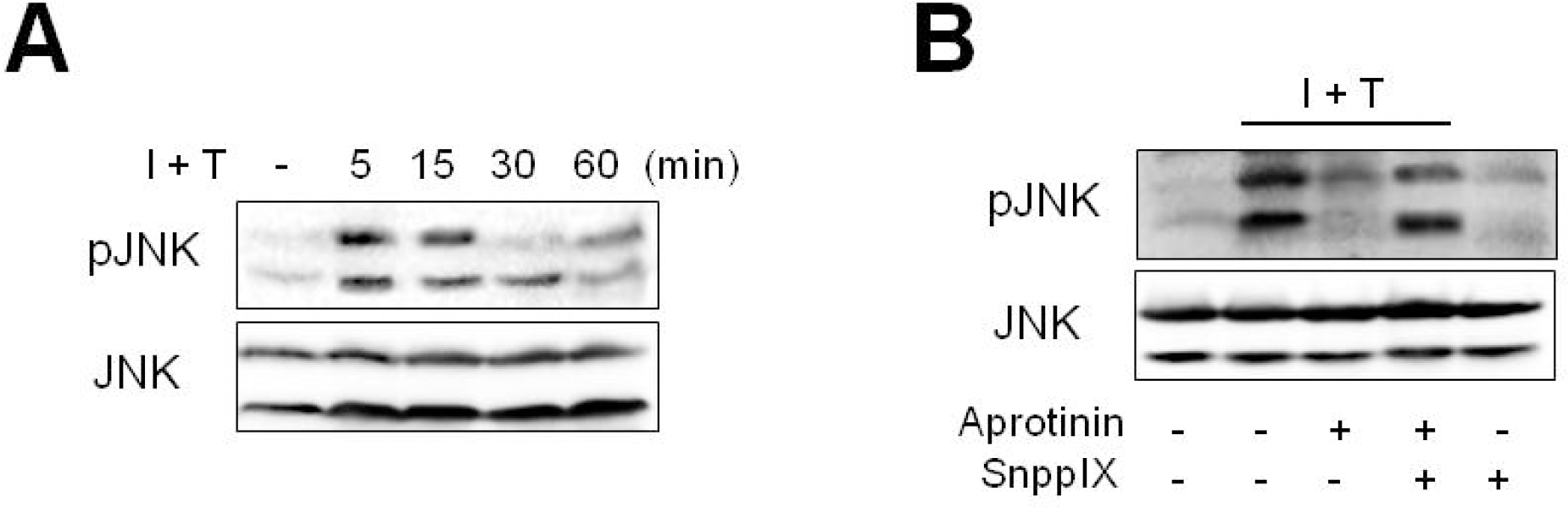
Aprotinin inhibits cytokine-induced JNK phosphorylation through HO-1. Phosphorylated JNK was measured in cells treated with cytokines (I+T) for 1 hr by Western blotting (A). Cells were pre-treated with aprotinin (10 μM) and SnPPIX (1 μM) for 1 hr, and were treated with cytokines for 5 minutes prior to measurement of phosphorylated JNK by Western blotting (B). Results are representative of three experiments.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download