Abstract
Recent studies have documented that testosterone relaxes several smooth muscles by modulating K+ channel activities. Smooth muscles of seminal vesicles play a fundamental role in ejaculation, which might involve testosterone. This study was aimed to assess the role of testosterone in seminal vesicular motility by studying its effects on contractile agents and on the ion channels of single vesicular myocytes in a rabbit model. The contractile responses of circular smooth muscle strips of rabbit seminal vesicles to norepinephrine (10μM), a high concentration of KCl (70 mM), and testosterone (10μM) were observed. Single vesicular myocytes of rabbit were isolated using proteolytic enzymes including collagenase and papain. Inside-out, attached, and whole-cell configurations were examined using the patch clamp technique. The applications of 10μM norepinephrine or 70 mM KCl induced tonic contractions, and 10μM testosterone (pharmacological concentration) evoked dose-dependent relaxations of these precontracted strips. Various K+ channel blockers, such as tetraethylammonium (TEA; 10 mM), iberiotoxin (0.1μM), 4-aminopyridine (4-AP, 10μM), or glibenclamide (10μM) rarely affected these relaxations. Single channel data (of inside-out and attached configurations) of BK channel activity were also hardly affected by testosterone (10μM). On the other hand, however, testosterone reduced L-type Ca2+ currents significantly, and found to induce acute relaxation of seminal vesicular smooth muscle and this was mediated, at least in part, by Ca2+ current inhibition in rabbit.
REFERENCES
Aumuller G., Riva A. Morphology and functions of the human seminal vesicle. Andrologia. 24:183–196. 1992.
Chou TM., Sudhir K., Hutchison SJ., Ko E., Amidon TM., Collins P., Chatterjee K. Testosterone induces dilation of canine coronary conductance and resistance arteries in vivo. Circulation. 94:2614–2619. 1996.

Cohen PG. The association of premature ejaculation and hypogonadotrophic hypogonadism. J Sex Marital Ther. 23:208–211. 1997.
Costarella CE., Stallone JN., Rutecki GW., Whittier FC. Testosterone causes direct relaxation of rat thoracic aorta. J Pharmacol Exp Ther. 277:34–39. 1996.
Deenadayalu VP., White RE., Stallone JN., Gao X., Garcia AJ. Testosterone relaxes coronary arteries by opening the large-conductance, calcium-activated potassium channel. Am J Physiol. 281:H1720–H1727. 2001.

Ding AQ., Stallone JN. Testosterone-induced relaxation of rat aorta is androgen structure specific and involves K+ channel activation. J Appl Physiol. 91:2742–2750. 2001.
Er F., Michels G., Brandt MC., Khan I., Hasse H., Eicks M., Lindner M., Hoppe UC. Impact of testosterone on cardiac L-type calcium channels and Ca2+ sparks: acute actions antagonize chronic effects. Cell Calcium. 41:467–477. 2007.
Hamill RW., Schroeder B. Hormonal regulation of adult sympathetic neurons: the effects of castration on neuropeptide Y, norepinephrine, and tyrosine hydroxylase activity. J Neurobiol. 21:731–742. 1990.

Hib J., Ponzio R., Gomez DE., Vilar O. Contractile behaviour of rat seminal vesicle after gonadectomy, testosterone replacement and cyproterone treatment. Andrologia. 17:435–439. 1985.

Kashiwagi B., Shibata Y., Ono Y., Suzuki K., Honma S., Yamanaka H. Effect of androgen concentration on seminal vesicle blood flow in rats-establishment of new highly sensitive simultaneous androgen measurement method. Urology. 66:218–223. 2005.

Kim J., Cole D., Johnson A., Centenera V., Schenkman E., Durham J., Azzaro A., Mawhinney M. Androgen induced norepinephrine release from postganglionic neurons mediates accessory sex organ smooth muscle proliferation. Urology. 167:1897–1904. 2002.

Melvin JE., McNeill TH., Hamill RW. Biochemical and morphological effects of castration on the postorganizational development of the hypogastric ganglion. Dev Brain Res. 38:131–139. 1988.

Motofei IG. A bihormonal model of normal sexual stimulation; the etiology of premature ejaculation. Med Hypotheses. 57:93–95. 2001.

Perusquia M., Navarrete E., Jasso-Kamel J., Montano LM. Androgens induce relaxation of contractile activity in pregnant human myometrium at term: a nongenomic action on L-type calcium channels. Biol Reprod. 73:214–221. 2005.
Scragg JL., Jones RD., Channer KS., Jones TH., Peers C. Testosterone is a potent inhibitor of L-type Ca2+ channels. Biochem Biophys Res Commun. 318:503–506. 2004.
Fig. 1.
Relaxation responses of circular smooth muscle strips of rabbit seminal vesicle. The IC50 was 1.49×10−6±4.43×10−7 M (n=15).

Fig. 2.
Typical representations of contractile responses of circular smooth muscle strips of rabbit seminal vesicle. 10μM norepinephrine (A) and high concentrations of KCl (70 and 140 mM; B) evoked sustained contractile responses. W/O, washout.

Fig. 3.
Effect of testosterone on norepinephrine- and KCl-induced contractions. 10μM norepinephrine and 70 mM KCl were used to evoke a precontracted status. 10μM testosterone evoked powerful and significant relaxations of both precontracted strips (n=8). Asterisks indicate p<0.05 versus control group.
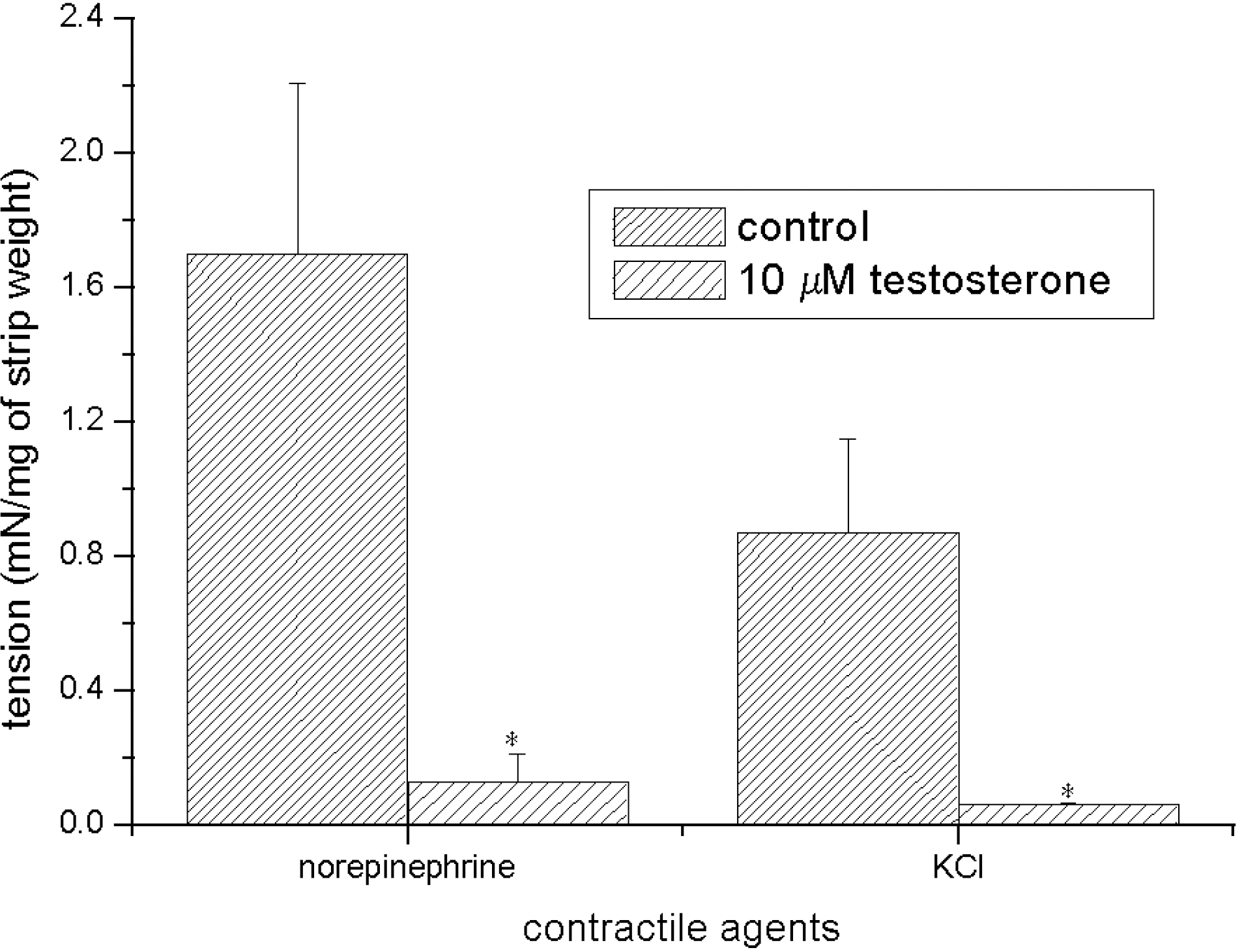
Fig. 4.
Effect of TEA incubation on the testosterone-induced relaxation. Following incubation with 1 mM or 10 mM TEA and the strips were precontracted with 70 mM KCl, 10μM TES still relaxed the strips (n=8). Asterisks indicate p<0.05 versus control group.

Fig. 5.
Effect of 10μM testosterone application on L-type Ca2+ channel currents. L-type Ca2+ channel currents appeared by depolarizing pulses from holding potentials of −50 mV (A). The currents were definitely decreased by 10μM testosterone application (B). Effect of 10μM testosterone on the current-voltage relations. (C) were obtained from a total of 7 smooth muscle cells of rabbit seminal vesicle. Depolarized conditions at above 0 mV testosterone significantly reduced the currents. Asterisks indicate p<0.05 versus control group.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download