Abstract
The present study examined the inhibitory effect of licorice compounds glycyrrhizin and a metabolite 18β-glycyrrhetinic acid on the neurotoxicity of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) in the mouse and on the 1-methyl-4-phenylpyridinium (MPP+)-induced cell death in differentiated PC12 cells. MPTP treatment increased the activities of total superoxide dismutase, catalase and glutathione peroxidase and the levels of malondialdehyde and carbonyls in the brain compared to control mouse brain. Co-administration of glycyrrhizin (16.8 mg/kg) attenuated the MPTP effect on the enzyme activities and formation of tissue peroxidation products. In vitro assay, licorice compounds attenuated the MPP+-induced cell death and caspase-3 activation in PC12 cells. Glycyrrhizin up to 100μM significantly attenuated the toxicity of MPP+. Meanwhile, 18β-glycyrrhetinic acid showed a maximum inhibitory effect at 10μM; beyond this concentration the inhibitory effect declined. Glycyrrhizin and 18β-glycyrrhetinic acid attenuated the hydrogen peroxide- or nitrogen species-induced cell death. Results from this study indicate that glycyrrhizin may attenuate brain tissue damage in mice treated with MPTP through inhibitory effect on oxidative tissue damage. Glycyrrhizin and 18β-glycyrrhetinic acid may reduce the MPP+ toxicity in PC12 cells by suppressing caspase-3 activation. The effect seems to be ascribed to the antioxidant effect.
Go to : 
REFERENCES
Aebi H. Catalase in vitro. Methods Enzymol. 105:121–126. 1984.
Agarwal MK., Iqbal M., Athar M. Inhibitory effect of 18β-glycyrrhetinic acid on 12-O-tetradecanoyl phorbol-13-acetate- induced cutaneous oxidative stress and tumor promotion in mice. Redox Rep. 10:151–157. 2005.
Bonuccelli U., Del Dotto P. New pharmacological horizons in the treatment of Parkinson disease. Neurology. 67(7 Suppl. 2):S30–S38. 2006.
Cassarino DS., Fall CP., Swerdlow RH., Smith TS., Halvorsen EM., Miller SW., Parks JP., Parker Jr WD., Bennet Jr JP. Elevated reactive oxygen species and antioxidant enzyme activities in animal and cellular models of Parkinson's disease. Biochim Biophys Acta. 1362:77–80. 1997.

Cassarino DS., Parks JK., Parker WD Jr., Bennett JP Jr. The Parkinsonian neurotoxin MPP+ opens the mitochondrial permeability transition pore and releases cytochrome c in isolated mitochondria via an oxidative mechanism. Biochim Biophys Acta. 1453:49–62. 1999.
Fleury C., Mignotte B., Vayssière J-L. Mitochondrial reactive oxygen species in cell death signaling. Biochimie. 84:131–141. 2002.

Flohe L., Gunzler WA. Assays of glutathione peroxidase. Methods Enzymol. 105:114–121. 1984.
Gutteridge JMC., Rowley DA., Halliwell B. Superoxide dependent formation of hydroxyl radicals and lipid peroxidation in the presence of iron salts. Detection of ‘catalytic’ iron and antioxidant activity in extracellular fluids. Biochem J. 206:605–609. 1982.
Hung HC., Lee EHY. MPTP produces differential oxidative stress and antioxidative responses in the nigrostriatal and mesolimbic dopaminergic pathways. Free Radic Biol Med. 24:76–84. 1998.

Ishiwata S., Nakashita K., Ozawa Y., Niizeki M., Nozaki S., Tomioka Y., Mizugaki M. Fas-mediated apoptosis is enhanced by glycyrrhizin without alteration of caspase-3-like activity. Biol Pharm Bull. 22:1163–1166. 1999.

Jeong HG., You HJ., Park SJ., Moon AR., Chung YC., Kang SK., Chun HK. Hepatoprotective effects of 18β-glycyrrhetinic acid on carbon tetrachloride-induced liver injury: inhibition of cytochrome P450 2E1 expression. Pharmacol Res. 46:221–227. 2002.

Kadota T., Yamaai T., Saito Y., Akita Y., Kawashima S., Moroi K., Inagaki N., Kadota K. Expression of dopamine transporter at the tips of growing neurites of PC12 cells. J Histochem Cytochem. 44:989–996. 1996.

Kim R., Emi M., Tanabe K. Role of mitochondria as the gardens of cell death. Cancer Chemother Pharmacol. 57:545–553. 2006.

Kinjo J., Hirakawa T., Tsuchihashi R., Nagao T., Okawa M., Nohara T., Okabe H. Hepatoprotective constituents in plants. 14. Effects of soyasapogenol B, sophoradiol, and their glucuronides on the cytotoxicity of tert-butyl hydroperoxide to HepG2 cells. Biol Pharm Bull. 26:1357–1360. 2003.
Lee CS., Kim YJ., Ko HH., Han ES. Modulation of 1-methyl-4-phenylpyridinium-induced mitochondrial dysfunction and cell death by KATP channel block. J Neural Transm. 114:297–305. 2007.
Lee DH., Han YS., Han ES., Bang H., Lee CS. Differential involvement of intracellular Ca2+ in 1-methyl-4-phenylpyridinium- or 6-hydroxydopamine-induced cell viability loss in PC12 cells. Neurochem Res. 31:851–860. 2006.
Levine RL., Garland D., Oliver CN., Amici A., Climent I., Lenz A-G., Ahn B-W., Shaltiel S., Stadman ER. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 186:464–478. 1990.
Makino T., Tsubouchi R., Murakami K., Haneda M., Yoshino M. Generation of reactive oxygen species and induction of apoptosis of HL60 cells by ingredients of traditional herbal medicine, Sho-saiko-to. Basic Clin Pharmacol Toxicol. 98:401–415. 2006.

Matsui S., Matsumoto H., Sonoda Y., Ando K., Aizu-Yokota E., Sato T., Kasahara T. Glycyrrhizin and related compounds down-regulate production of inflammatory chemokines IL-8 and eotaxin 1 in a human lung fibroblast cell line. Int Immunopharmacol. 15:1633–1644. 2004.

McCord JM., Fridovich I. Superoxide dismutase. Enzymatic function for erythrocuprein (hemocuprein). J Biol Chem. 244:6049–6055. 1969.
Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 65:55–63. 1983.

Muijsers RBR., Folkerts G., Henricks PA., Sadeghi-Hashjin G., Nijkamp FP. Peroxynitrite: a two faced metabolite of nitric oxide. Life Sci. 60:1833–1845. 1997.
Nagai T., Egashira T., Yamanaka Y., Kohno M. The protective effect of glycyrrhizin against injury of the liver caused by ischemia-reperfusion. Arch Environ Contam Toxicol. 20:432–436. 1991.

Olanow CW., Tatton WG. Etiology and pathogenesis of Parkinson's disease. Annu Rev Neurosci. 22:123–144. 1999.

Przedborski S., Jackson-Lewis V. Mechanism of MPTP toxicity. Mov Disord. 13:35–38. 1998.
Schulz JB., Matthews RT., Klockgether T., Dichgans J., Beal MF. The role of mitochondrial dysfunction and neuronal nitric oxide in animal models of neurodegenerative diseases. Mol Cell Biochem. 174:193–197. 1997.

Shibata S. A drug over the millennia: pharmacognosy, chemistry, and pharmacology of licorice. Yakugaku Zasshi. 120:849–862. 2000.
Tatton WG., Chalmers-Redman RME., Ju WJH., Mammen M., Carlile GW., Pong AW., Tatton NA. Propargylamines induce antiapoptotic new protein synthesis in serum-and nerve growth factor (NGF)-withdrawn, NGF-differentiated PC-12 cells. J Pharmacol Exp Ther. 301:753–764. 2002.
Yokozawa T., Liu ZW., Chen CP. Protective effects of Glycyrrhizae radix extract and its compounds in a renal hypoxia (ischemia)-reoxygenation (reperfusion) model. Phytomedicine. 6:439–445. 2000.
Yoshikawa M., Toyohara M., Ueda S., Shirol A., Takeuchi H., Nishiyama T., Yamada T., Fukui H., Ishizaka S. Glycyrrhizin inhibits TNF-induced, but not Fas-mediated, apoptosis in the human hepatoblastoma line HepG2. Biol Pharm Bull. 22:951–955. 1999.

Zheng QZ., Lou YJ. Pathologic characteristics of immunologic injury in primary cultured rat hepatocytes and protective effect of glycyrrhizin in vitro. Acta Pharmacol Sin. 24:771–777. 2003.
Go to : 
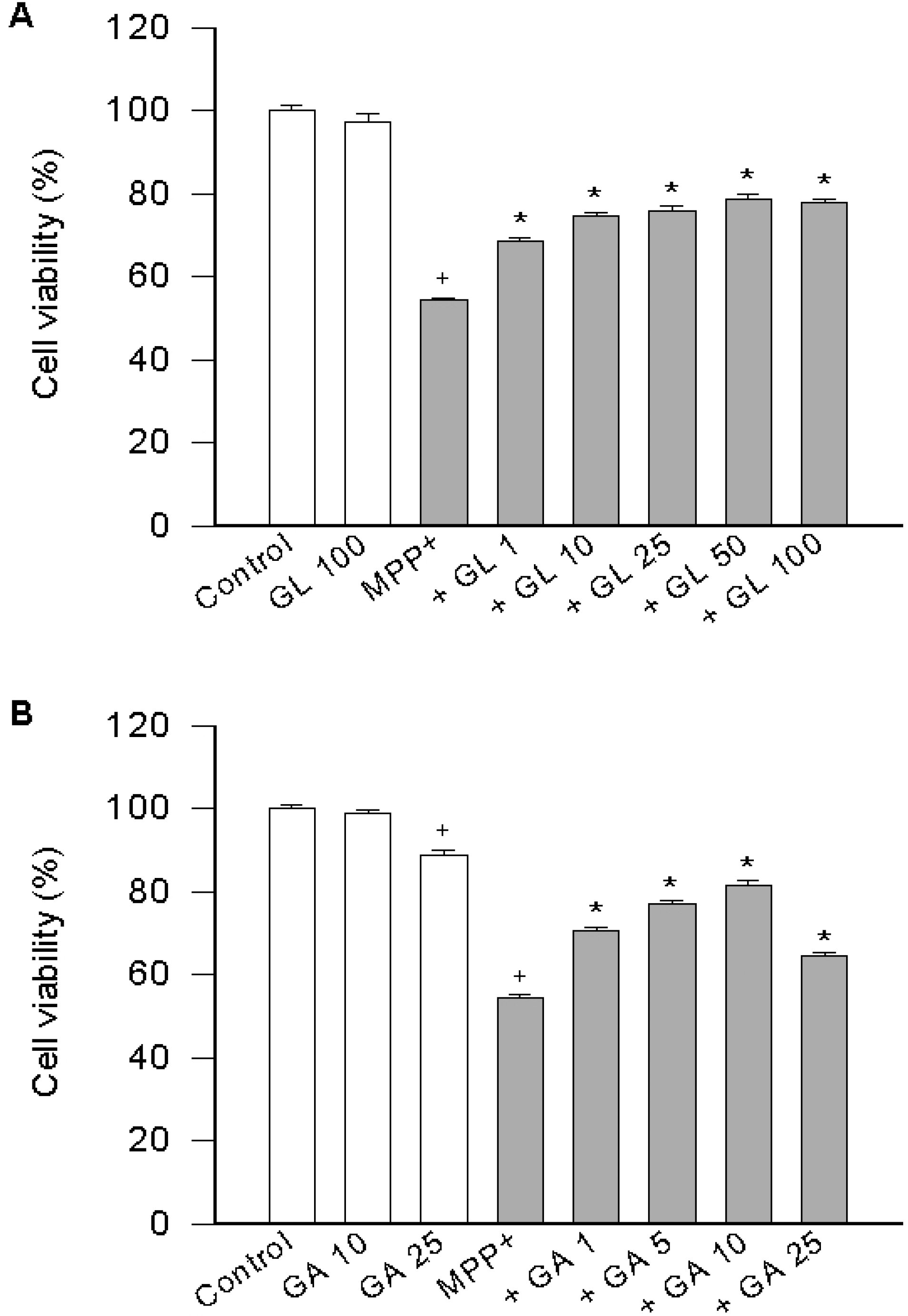 | Fig. 1.Effect of licorice compounds on MPP+-induced cell death. PC12 cells were pre-treated with licorice compounds (1~100 μM GL in A or 1-25 μM GA in B) for 20 min, exposed to 500 μM MPP+ for 24 h and cell viability was determined. Data represent means ± SEM (n=6). +p<0.05 compared to control (percentage of control); and ∗p<0.05 compared to MPP+ alone. |
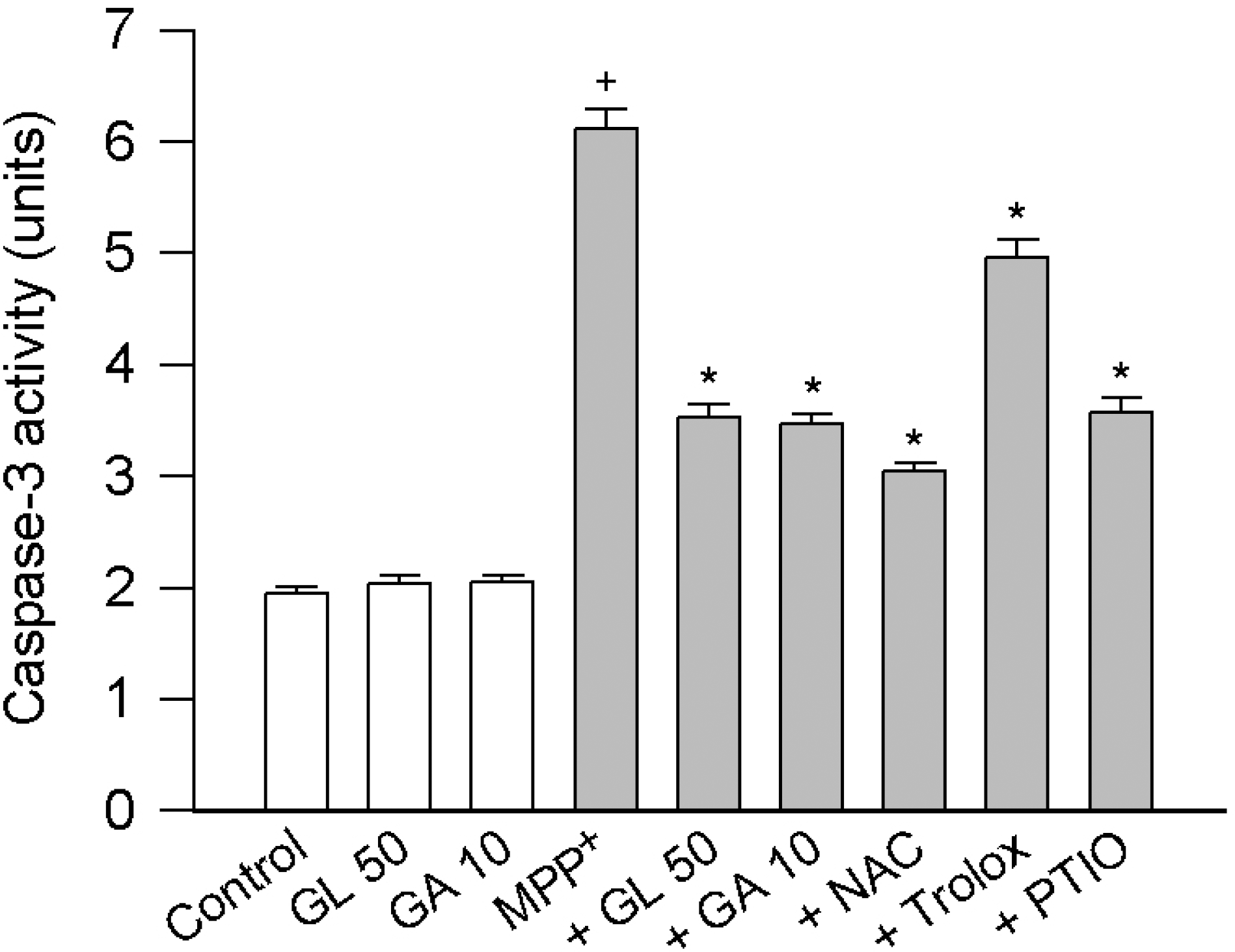 | Fig. 2.Effect of licorice compounds on MPP+-induced activation of caspase-3. PC12 cells were treated with 500 μM MPP+ in the presence of licorice compounds (10~50 μM) or scavengers [1 mM N-acetylcysteine (NAC), 30 μM trolox or 30 μM carboxy-PTIO (PTIO)] for 24 h. Data are expressed as units for caspase-3 activity and represent means ± SEM (n=6). +p<0.05 compared to control; and ∗p<0.05 compared to MPP+ alone. |
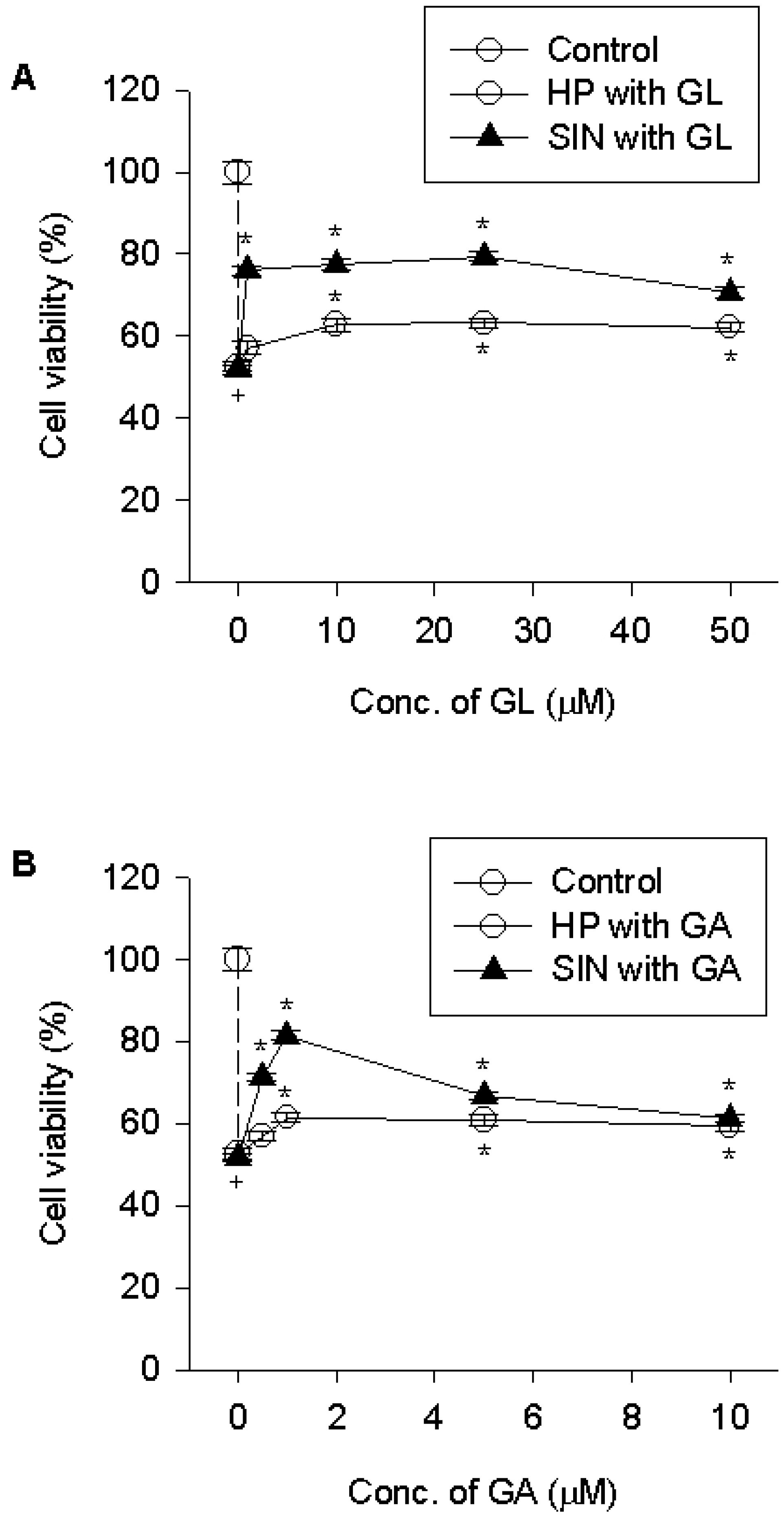 | Fig. 3.Effect of licorice compounds on cell death due to hydrogen peroxide or 3-morpholinosydnonime. PC12 cells were pre-treated with licorice compounds [1~50 μM GL (A) or 1~10 μM GA (B)] for 15 min, exposed to 200 μM hydrogen peroxide for 4 h or 750 μM 3-morpholinosydnonime for 24 h, and cell viability was determined. Data represent means ± SEM (n=6). +p<0.05 compared to control; and ∗p<0.05 compared to hydrogen peroxide (HP) or 3-morpholinosydnonime (SIN). |
Table 1.
Effect of GL on increased antioxidant enzyme activities in the brains of mice treated with MPTP
| Control | MPTP | MPTP+GL | GL | |
|---|---|---|---|---|
| BDM | ||||
| SOD | 23.16±0.73 | 39.32±1.19† | 28.86±0.96∗ | 24.58±0.81 |
| Catalase | 0.603±0.024 | 0.926±0.025† | 0.723±0.023∗ | 0.603±0.022 |
| GSH peroxidase | 3.999±0.111 | 6.269±0.173† | 5.003±0.172∗ | 4.276±0.136 |
| Cortex | ||||
| SOD | 7.98±0.22 | 9.95±0.31† | 8.94±0.22∗ | 7.78±0.23 |
| Catalase | 0.384±0.032 | 0.520±0.025† | 0.428±0.034∗ | 0.406±0.027 |
| GSH peroxidase | 3.698±0.129 | 4.984±0.145† | 4.120±0.129∗ | 3.778±0.146 |
| Cerebellum | ||||
| SOD | 11.84±0.47 | 17.23±0.67† | 13.66±0.75∗ | 11.43±0.45 |
| Catalase | 0.857±0.039 | 1.123±0.040† | 0.920±0.043∗ | 0.841±0.037 |
| GSH peroxidase | 5.145±0.256 | 7.014±0.117† | 5.908±0.266∗ | 5.366±0.198 |
Table 2.
Effect of GL on formation of MDA and carbonyls in the brains of mice treated with MPTP
| Control | MPTP | MPTP+GL | GL | |
|---|---|---|---|---|
| BDM | ||||
| MDA | 0.268±0.010 | 0.462±0.017† | 0.340±0.014∗ | 0.282±0.012 |
| Carbonyls | 1.835±0.059 | 2.918±0.102† | 2.248±0.117∗ | 1.907±0.079 |
| Cortex | ||||
| MDA | 0.292±0.012 | 0.384±0.019† | 0.328±0.013∗ | 0.285±0.014 |
| Carbonyls | 1.875±0.085 | 2.494±0.133† | 2.120±0.129∗ | 1.973±0.089 |
| Cerebellum | ||||
| MDA | 0.306±0.012 | 0.409±0.016† | 0.358±0.014∗ | 0.273±0.013 |
| Carbonyls | 1.971±0.085 | 2.705±0.090† | 2.238±0.103∗ | 1.890±0.082 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download