Abstract
This study was carried out to investigate the wound healing effect of caffeic acid in skin-incised mice. Caffeic acid showed significant effects on anti-inflammatory activity and wound healing, such as myeloperoxidase activity, lipid peroxidation, phospholipase A2 activity and collagen-like polymer synthesis, in incised-wound tissue. On the other hand, it significantly stimulated collagen-like polymer synthesis in NIH 3T3 fibroblast cells, while inhibited both silica-induced reactive oxygen species generation and melittin-induced arachidonic acid release and PGE2 production in Raw 264.7 cells, and histamine release in RBL 2H3 cells stimulated by melittin or arachidonic acid. Therefore, caffeic acid appears to have a potent antioxidant and anti-inflammatory effect in cell culture system, which may be related to wound healing in skin-incised mice.
Go to : 
REFERENCES
Balboa MA., Balsinde J., Johnson CA., Dennis EA. Regulation of arachidonic acid mobilization in lipopolysaccharide-activated P388D(1) macrophages by adenosine triphosphate. J Biol Chem. 17:36764–36768. 1999.

Bodeker G., Hughes MA. Wound healing, traditional treatments and research policy. Prendergast HDV, Etkin NL, Harris DR, Houghton PJ, editors. ed,. Plants for Food and Medicine. 1st ed.Royal Botanic Gardens Kew;London: p. p. 345–359. 1998.
Boland A., Delapierre D., Mossay D., Hans P., Dresse A. Propofol protects cultured brain cells from iron ion-induced death: comparison with trolox. Eur J Pharmacol. 404:21–27. 2000.

Bradley PP., Priebat DA., Christensen RD., Rothstein G. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J Invest Dermatol. 78:206–209. 1982.

Clark RAF. Biology of dermal wound repair. Nemeth AJ, editor. ed,. Dermatologic Clinics. Elsevier;Philadelphia, PA: ETATS-UNIS. Wound Healing. Vol. 11:p. p. 647–666. 1993.

Gan XL., Hei ZQ., Huang HQ., Chen LX., Li SR., Cai J. Effect of Astragalus membranaceus injection on the activity of the intestinal mucosal mast cells after hemorrhagic shock-reperfusion in rats. Chin Med. 119:1892–1898. 2006.

Gupta A., Singh RL., Raghubir R. Antioxidant status during cutaneous wound healing in immunocompromised rats. Mol Cell Biochem. 241:1–7. 2002.
James TJ., Hughes MA., Hofman D., Cherry GW., Taylor RP. Antioxidant characteristics chronic wound fluid. Br J Dermatol. 145:185–186. 2001.
Laiho K. Myeloperoxidase activity in skin lesions. I. Influence of the loss of blood, depth of excoriations and thickness of the skin. Int J Legal Med. 111:6–9. 1998.
Lamme EN., Van Leeuwen RTJ., Brandsma K., Van Marle J., Middelkoop E. Higher numbers of autologous fibroblasts in an artificial dermal substitute improve tissue regeneration and modulate scar tissue formation. J Pathol. 190:595–603. 2000.

Mensah AY., Sampson J., Houghton PJ., Hylands PJ., Westbrook J., Dunn M., Hughes MA., Cherry GW. Effects of Buddleja globosa leaf and its constituents relevant to wound healing. J Ethnopharmacol. 77:219–226. 2001.

Mills TA., Taggart MJ., Greenwood SL., Baker PN., Wareing M. Histamine-induced contraction and relaxation of placental chorionic plate arteries. Placenta. 28:1158–1164. 2007.

Natarajan K., Singh S., Burke TR., Grunberger D., Aggarwal BB. Caffeic acid phenethyl ester is a potent and specific inhibitor of activation of nuclear transcription factor NF-κB. Nat Acad Sci. 93:9090–9095. 1996.
Pascual C., Gonzalez R., Torricella RG. Scavenging action of propolis extract against oxygen radicals. J Ethnopharmacol. 41:9–13. 1994.

Radvanyi F., Jordan L., Russo-Marie F., Bon CA. Sensitive and continuous flurometric assay for phospholipase A2 using pyren-labeled phospholipids in the presence of serum albumin. Anal Biochem. 177:103–109. 1989.
Russo A., Longo R., Vanella A. Antioxidant activity of propolis: role of caffeic acid phenethyl ester and galangin. Fitoterapia. 73:21–29. 2002.

Shore PA. Burkhalter A, Cohn VH. A method for the fluorometric assay of histamine in tissues. J Pharmacol Exp Ther. 127:182–186. 1959.
Smith PK., Krohn RI., Hermanson GT., Mallia AK., Gartner FH., Provenzano MD., Fujimoto EK., Goeke NM., Olson BJ., Klenk DC. Measurement of protein using bicinchoninic acid. Anal Biochem. 150:76–85. 1985.

Sud'ina GF., Mirzoeva OK., Pushkareva MA., Korshunova GA., Sumbatyan NV., Varfolomeev SD. Caffeic acid phenethyl ester as a lipoxygenase inhibitor with antioxidant properties. FEBS Lett. 329:21–24. 1993.
Suguna L., Singh S., Sivakumar P., Sampath P., Chandrakasan G. Influence of Terminalia chebula on dermal wound healing in rats. Phytother Res. 16:227–231. 2002.

Van Hien T., Hughes MA., Cherry GWC. In vitro studies on the antioxidant and growth stimulatory activities of a polyphenolic extract from Cudrania cochinchinensis used in the treatment of wounds in Vietnam. Wound Rep Reg. 5:159–167. 1997.

Go to : 
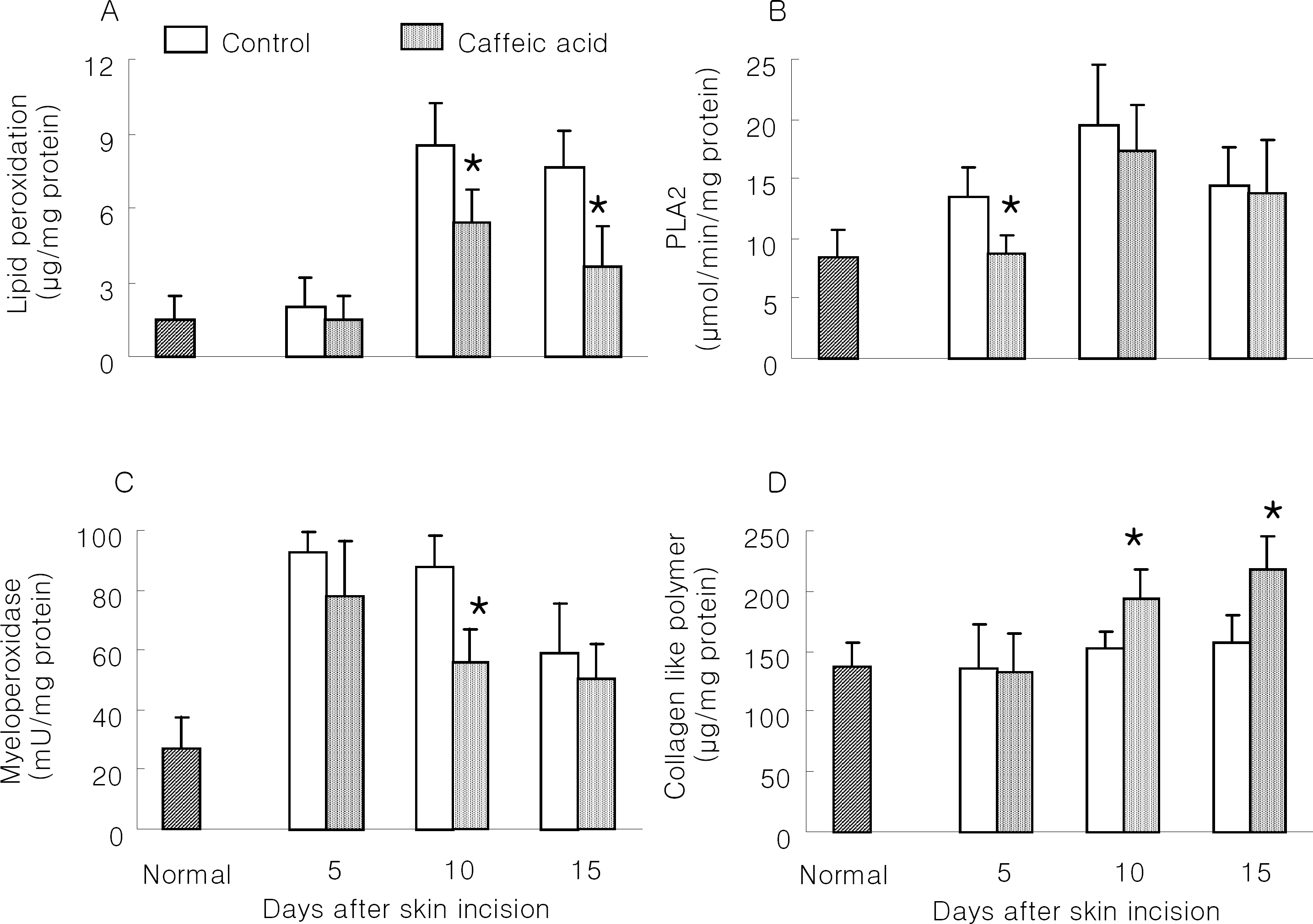 | Fig. 1.Effect of caffeic acid on wound healing in skin-incised mice. Two cm long incision wound perforating the skin was made on the dorsal skin of mice. Mice were orally treated daily with 1% carboxymethyl cellulose solution (CMC; Control) and 10 mg/kg caffeic acid in 1% CMC solution during the study period. On days 5, 10 and 15, zones of about 1mm in thickness were taken from the edges of the wounds for biochemical analysis, such as lipid peroxidation (A), phospholipase A2 (B), myeloperoxidase (C) and collagen-like polymer (D). Results are means±SD from 5 mice. ∗Significantly different from control (p<0.05). |
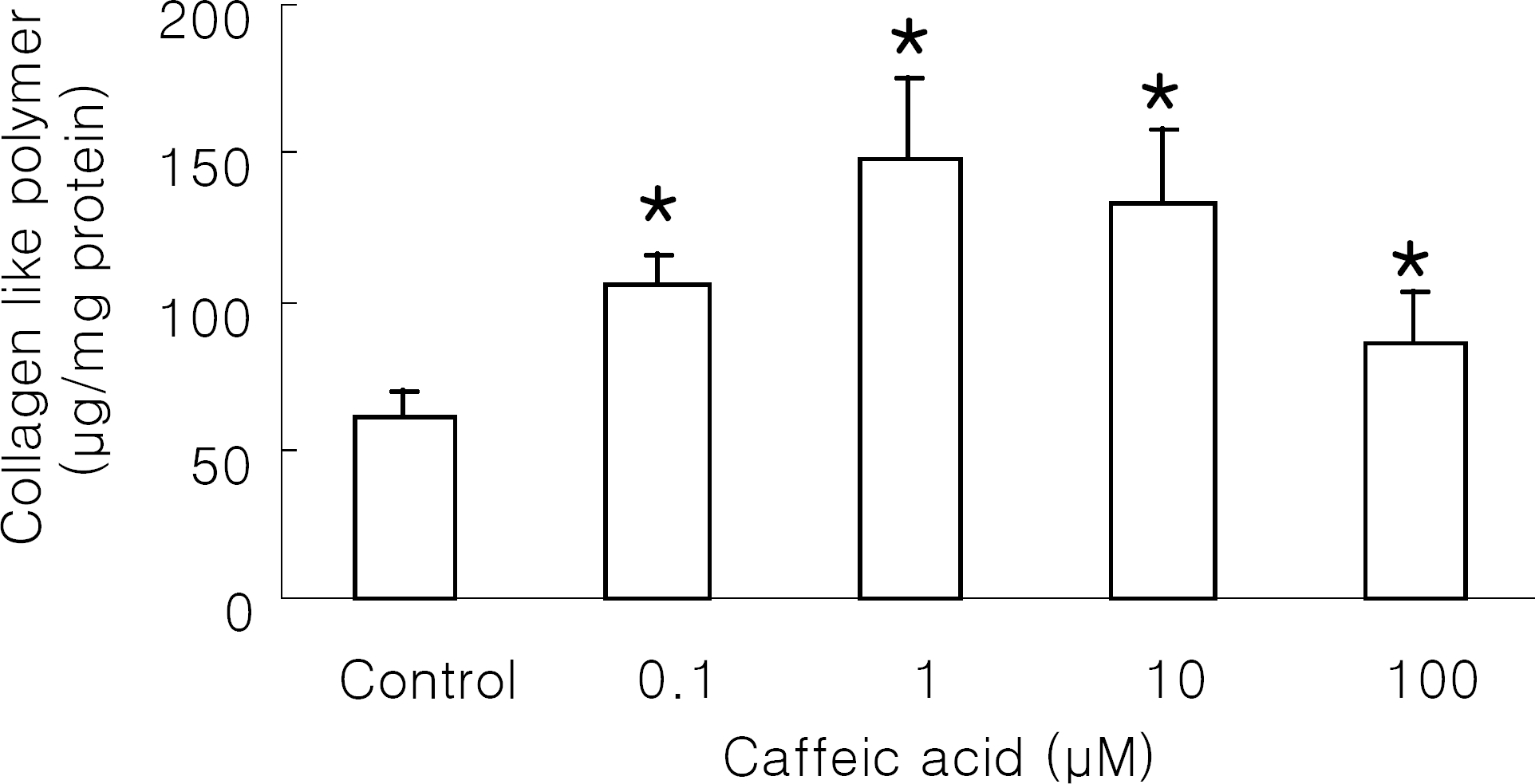 | Fig. 2.Effect of caffeic acid on collagen-like polymer production in NIH 3T3 cells. NIH 3T3 cells were incubated with caffeic acid at 37°C for 48 h. Control was the cells treated with 1% DMSO. Results are means±SD from 5 separate experiments. ∗Significantly different from control (p<0.05). |
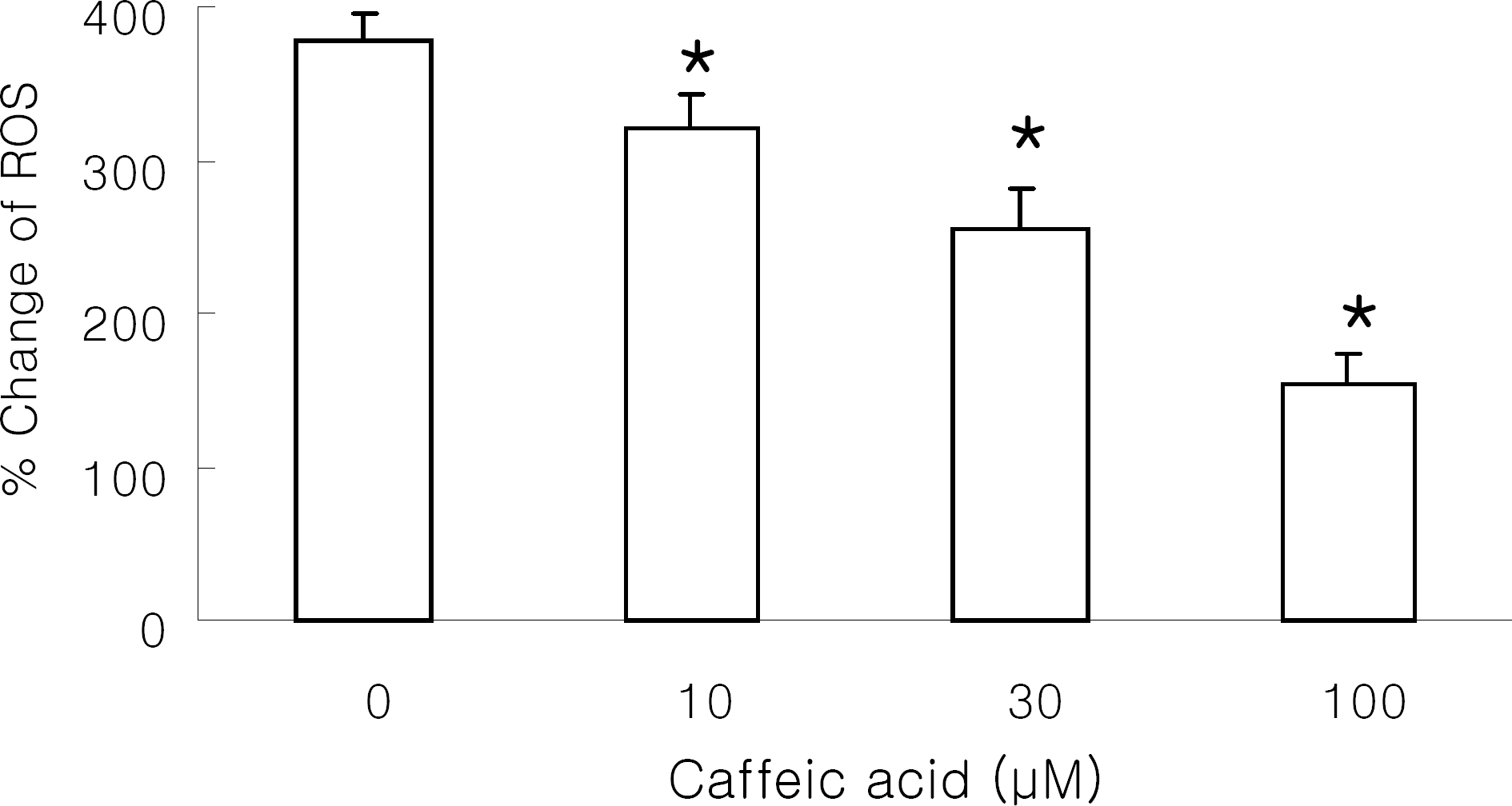 | Fig. 3.Effect of caffeic acid on reactive oxygen species (ROS) generation in Raw 264.7 cells. DCF-loaded Raw 264.7 cells were preincubated with caffeic acid and stimulated with 1 mg/ml silica at 37°C for 30 min. Results are means±SD from 5 separate experiments. ∗Significantly different from control (p<0.05). |
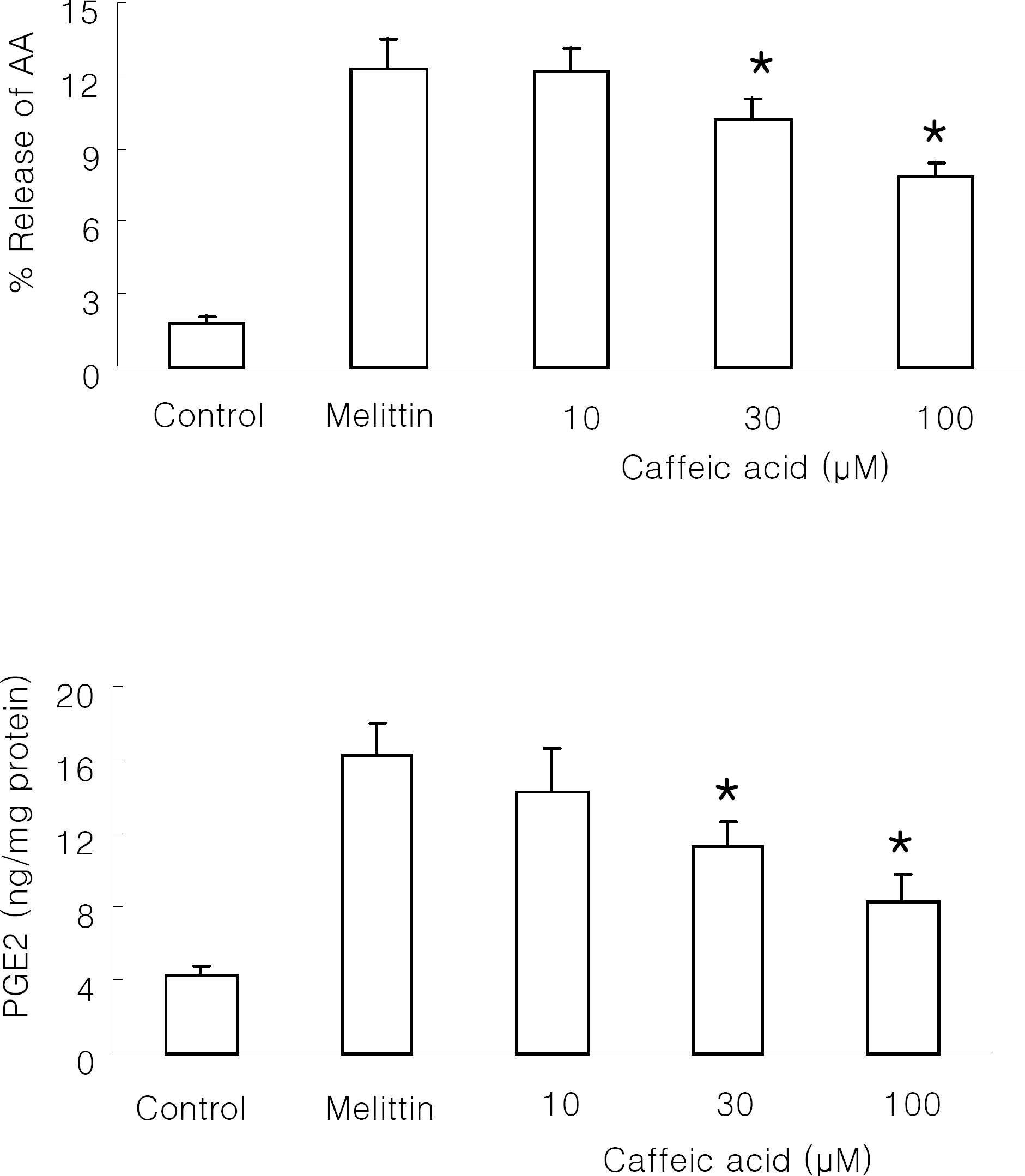 | Fig. 4.Effect of caffeic acid on melittin-induced arachidonic acid release and PGE2 production in Raw 264.7 cells. The cells were stimulated with 0.5 μM melittin in the presence or absence of caffeic acid at 37°C for 30 min. Control was treated with 1% DMSO in the absence of melittin. Intracellular amount of PGE2 was measured using PGE2 assay kit. Results are means±SD from 4 separate experiments. ∗Significantly different from control (p<0.05). |
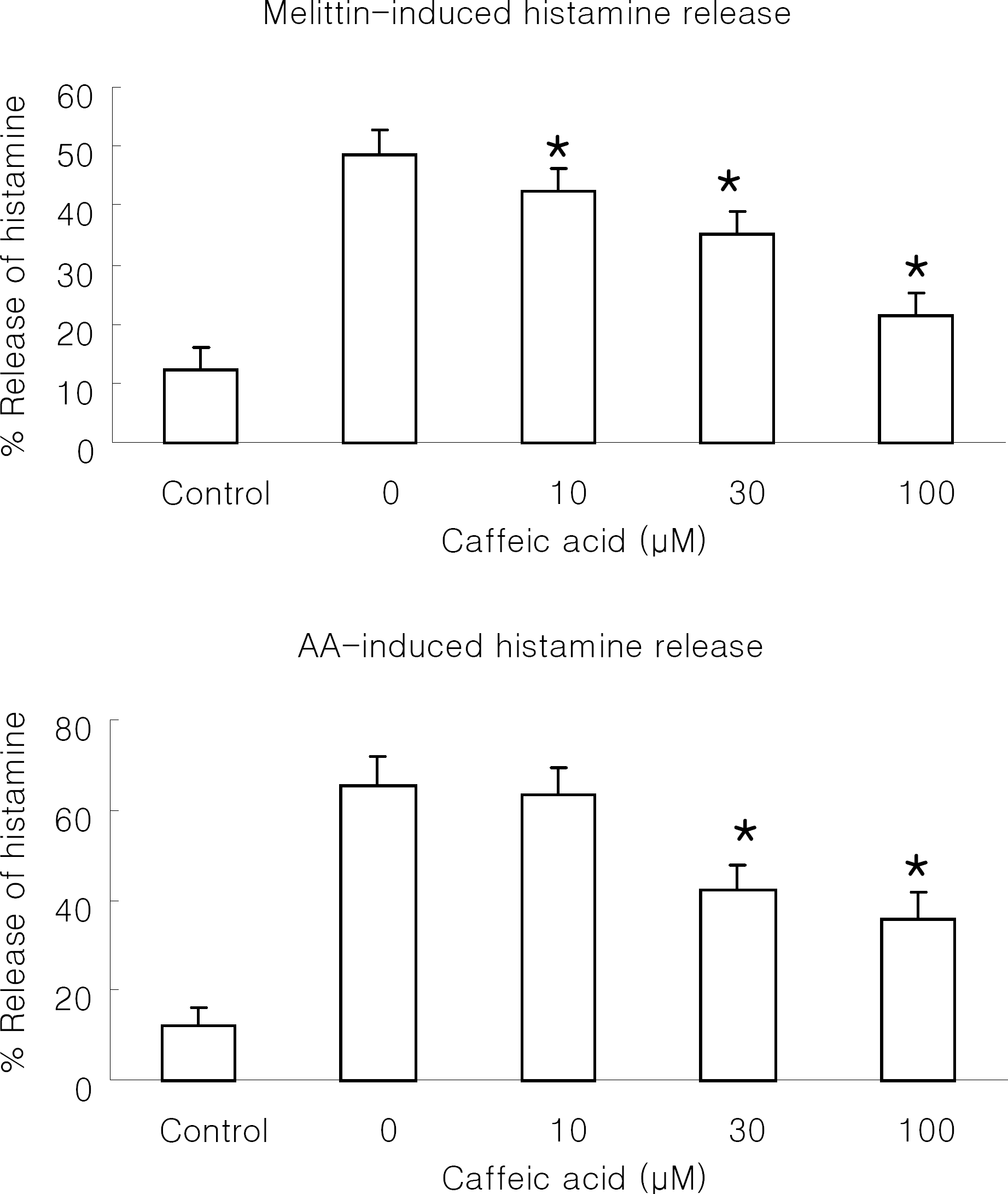 | Fig. 5.Effect of caffeic acid on melittin or arachidonic acid-induced histamine release in RBL 2H3 cells. The cells were stimulated with 0.5 μM melittin (A) or 100 μM arachidonic acid (AA; B) in the presence or absence of caffeic acid at 37°C for 30 min. Control was treated with 1% DMSO in the absence of melittin or arachidonic acid. Results are means±SD from 5 separate experiments. ∗Significantly different from control (p<0.05). |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download