Abstract
Eugenol is widely used in dentistry to relieve pain. We have recently demonstrated voltage-gated Na+ and Ca2+ channels as molecular targets for its analgesic effects, and hypothesized that eugenol acts on P2X3, another pain receptor expressed in trigeminal ganglion (TG), and tested the effects of eugenol by whole-cell patch clamp and Ca2+ imaging techniques. In the present study, we investigated whether eugenol would modulate 5'-triphosphate (ATP)-induced currents in rat TG neurons and P2×3-expressing human embryonic kidney (HEK) 293 cells. ATP-induced currents in TG neurons exhibited electrophysiological properties similar to those in HEK293 cells, and both ATP- and α, β-meATP-induced currents in TG neurons were effectively blocked by TNP-ATP, suggesting that P2×3 mediates the majority of ATP-induced currents in TG neurons. Eugenol inhibited ATP-induced currents in both capsaicin-sensitive and capsaicin-insensitive TG neurons with similar extent, and most ATP-responsive neurons were IB4-positive. Eugenol inhibited not only Ca2+ transients evoked by α, β-meATP, the selective P2×3 agonist, in capsaicin-insensitive TG neurons, but also ATP-induced currents in P2×3-expressing HEK293 cells without co-expression of transient receptor potential vanilloid 1 (TRPV1). We suggest, therefore, that eugenol inhibits P2×3 currents in a TRPV1-independent manner, which contributes to its analgesic effect.
Go to : 
REFERENCES
Alavi AM., Dubyak GR., Burnstock G. Immunohistochemical evidence for ATP receptors in human dental pulp. J Dent Res. 80:476–483. 2001.

Bleehen T., Keele CA. Observations on the algogenic actions of adenosine compounds on the human blister base preparation. Pain. 3:367–377. 1977.

Burnstock G. A unifying purinergic hypothesis for the initiation of pain. Lancet. 347:1604–1605. 1996.

Burnstock G., Wood JN. Purinergic receptors: their role in nociception and primary afferent neurotransmission. Curr Opin Neurobiol. 6:526–532. 1996.

Burnstock G. Purinergic P2 receptors as targets for novel analgesics. Pharmacol Ther. 110:433–454. 2006.

Caterina MJ., Leffler A., Malmberg AB., Martin WJ., Trafton J., Petersen-Zeitz KR., Koltzenburg M., Basbaum AI., Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 288:306–313. 2000.

Chen CC., Akopian AN., Sivilotti L., Colquhoun D., Burnstock G., Wood JN. A P2X purinoceptor expressed by a subset of sensory neurons. Nature. 377:428–431. 1995.

Chung G., Rhee JN., Jung SJ., Kim JS., Oh SB. Modulation of CaV2.3 calcium channel currents by eugenol. J Dent Res. 87:137–141. 2008.

Cook SP., McCleskey EW. Desensitization, recovery and Ca(2+)-dependent modulation of ATP-gated P2X receptors in nociceptors. Neuropharmacology. 36:1303–1308. 1997.

Cook SP., Vulchanova L., Hargreaves KM., Elde R., McCleskey EW. Distinct ATP receptors on pain-sensing and stretch-sensing neurons. Nature. 387:505–508. 1997.

Cook SP., McCleskey EW. Cell damage excites nociceptors through release of cytosolic ATP. Pain. 95:41–47. 2002.

Craig RG., Powers JM. Biocompatibility of dental materials. Restorative dental materials. St. Louis: Mosby;p. p. 154–155. 2002.
Dunn PM., Zhong Y., Burnstock G. P2X receptors in peripheral neurons. Prog Neurobiol. 65:107–134. 2001.

Eriksson J., Bongenhielm U., Kidd E., Matthews B., Fried K. Distribution of P2×3 receptors in the rat trigeminal ganglion after inferior alveolar nerve injury. Neurosci Lett. 254:37–40. 1998.
Gerevich Z., Zadori Z., Müller C., Wirkner K., Schröder W., Rubini P., Illes P. Metabotropic P2Y receptors inhibit P2×3 receptor-channels via G protein-dependent facilitation of their desensitization. Br J Pharmacol. 151:226–236. 2007.
Guo A., Vulchanova L., Wang J., Li X., Elde R. Immunocytochemical localization of the vanilloid receptor 1 (VR1): relationship to neuropeptides, the P2×3 purinoceptor and IB4 binding sites. Eur J Neurosci. 11:946–958. 1999.
Ichikawa H., Sugimoto T. The co-expression of P2×3 receptor with VR1 and VRL-1 in the rat trigeminal ganglia. Brain Res. 998:130–135. 2004.
Jiang J., Gu J. Expression of adenosine triphosphate P2×3 receptors in rat molar pulp and trigeminal ganglia. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 94:622–626. 2002.
Kollarik M., Dinh QT., Fischer A., Undem BJ. Capsaicin-sensitive and -insensitive vagal bronchopulmonary C-fibres in the mouse. J Physiol. 551:869–879. 2003.

Lee MH., Yeon KY., Park CK., Li HY., Fang Z., Kim MS., Choi SY., Lee SJ., Lee S., Park K., Lee JH., Kim JS., Oh SB. Eugenol inhibits calcium currents in dental afferent neurons. J Dent Res. 84:848–851. 2005.

Lewis C., Neidhart S., Holy C., North RA., Buell G., Surprenant A. Coexpression of P2×2 and P2×3 receptor subunits can account for ATP-gated currents in sensory neurons. Nature. 377:432–435. 1995.
Li HY., Oh SB., Kim JS. Pharmacological and electrophysiological characterization of rat P2X currents. IJOB. 33:1–5. 2008.
Li HY., Park CK., Jung SJ., Choi SY., Lee SJ., Park K., Kim JS., Oh SB. Eugenol inhibits K+ currents in trigeminal ganglion neurons. J Dent Res. 86:898–902. 2007.

Luo J., Yin GF., Gu YZ., Liu Y., Dai JP., Li C., Li ZW. Characterization of three types of ATP-activated current in relation to P2X subunits in rat trigeminal ganglion neurons. Brain Res. 1115:9–15. 2006.

North RA. The P2×3 subunit: a molecular target in pain therapeutics. Curr Opin Investig Drugs. 4:833–840. 2003.
North RA. P2×3 receptors and peripheral pain mechanisms. J Physiol. 554:301–308. 2004.
Park CK., Li HY., Yeon KY., Jung SJ., Choi SY., Lee SJ., Lee S., Park K., Kim JS., Oh SB. Eugenol inhibits sodium currents in dental afferent neurons. J Dent Res. 85:900–904. 2006.

Ralevic V., Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 50:413–492. 1998.
Renton T., Yiangou Y., Baecker PA., Ford AP., Anand P. Capsaicin receptor VR1 and ATP purinoceptor P2×3 in painful and nonpainful human tooth pulp. J Orofac Pain. 17:245–250. 2003.
Sarrami N., Pemberton MN., Thornhill MH., Theaker ED. Adverse reactions associated with the use of eugenol in dentistry. Br Dent J. 193:257–259. 2002.

Tsuda M., Ueno S., Inoue K. In vivo pathway of thermal hyperalgesia by intrathecal administration of alpha,beta-methylene ATP in mouse spinal cord: involvement of the glutamate-NMDA receptor system. Br J Pharmacol. 127:449–456. 1999.
Go to : 
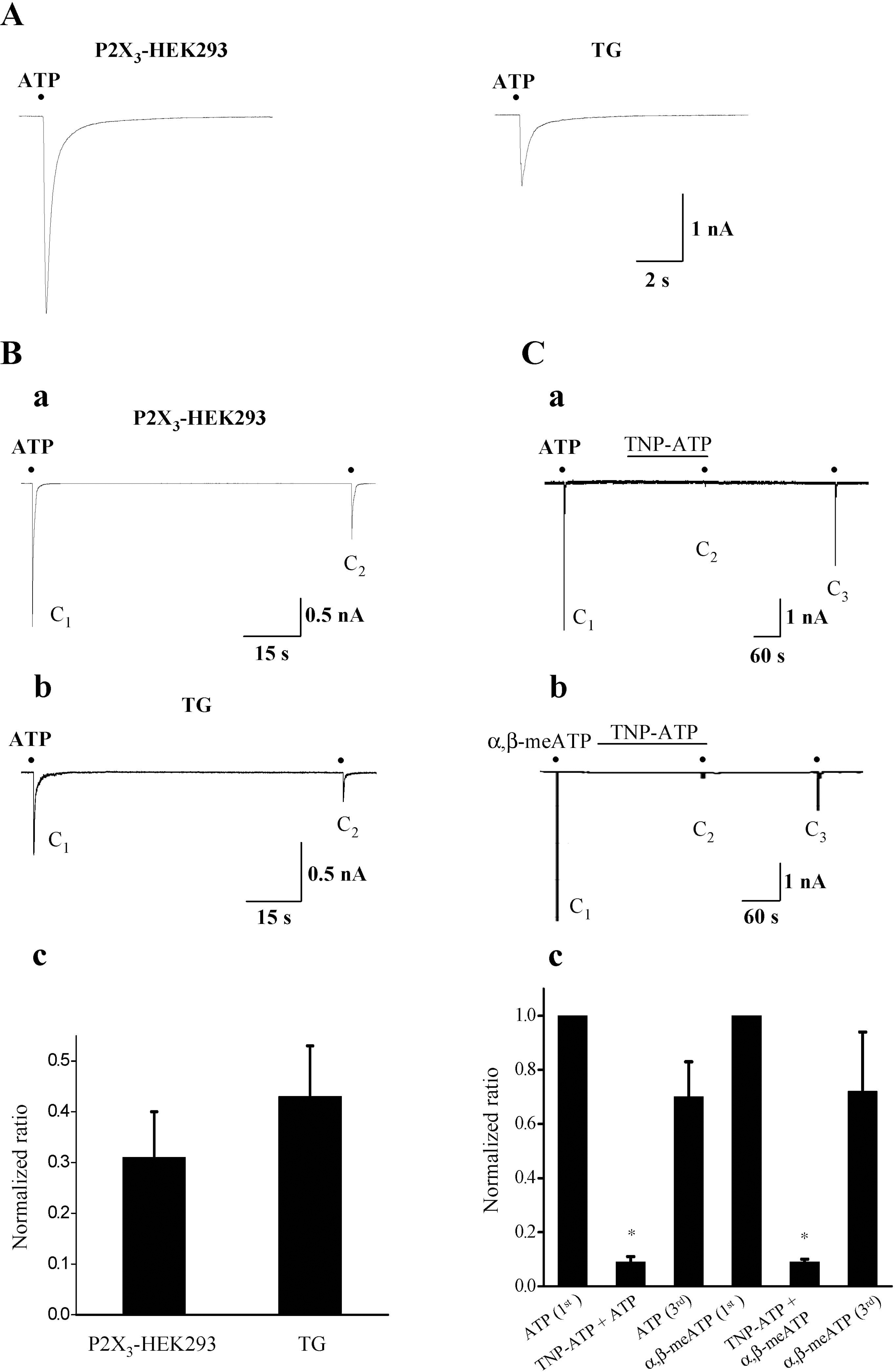 | Fig. 1.Comparison of current profiles of 10 μM ATP-induced currents in P2X3-expressing HEK293 cells and rat TG neurons. (A) Representative current traces activated by 10 μM ATP in P2X3-expressing HEK293 cells (left, n=60) and rat TG neurons (right, n=56). (B) Records of 10 μM ATP-induced currents activated by twice applications of 10 μM ATP with the interval of 90s in a P2X3-expressing HEK293 cell (a) and rat TG neuron (b). The normalized amplitude ratio (C2/C1) in P2X3-expressing HEK293 cells and that in TG neurons (c). (C) Inhibition of 10 μM ATP (a)- and 100 μM α, β-meATP (b)-induced currents by 1 μM TNP-ATP in small TG neurons. The summary of the inhibition of TNP-ATP on ATP (n=5)- and α, β-meATP (n=5)-induced currents (mean±SEM, p<0.05) (c). Black points indicate the time point of ATP or α,β-meATP application. |
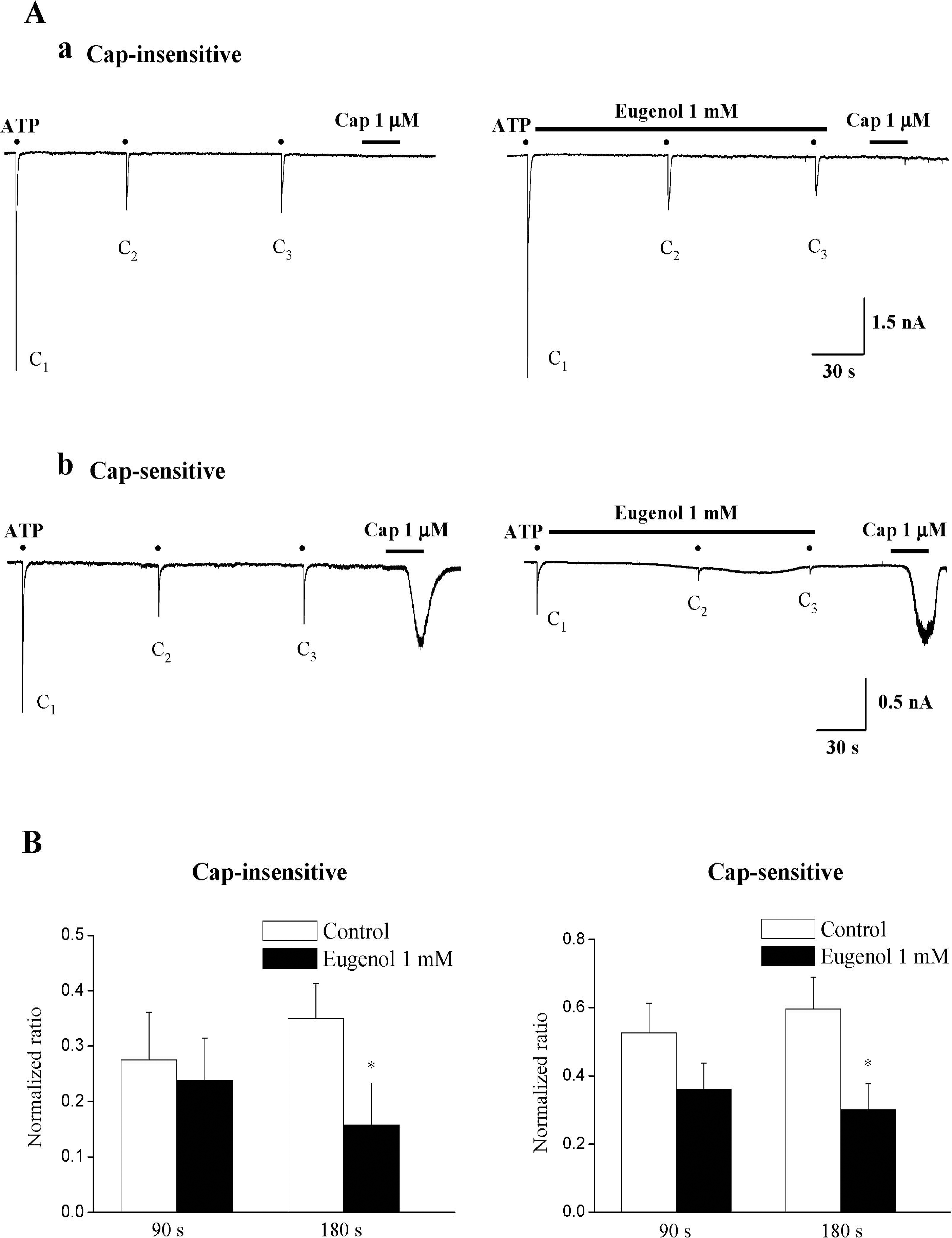 | Fig. 2.Eugenol inhibited ATP-induced P2X currents in both capsaicin-insensitive and capsaicin-sensitive rat TG neurons. (A) Representative current traces of 10 μM ATP-induced P2X currents under control (Aa and Ab, left), and eugenol (1 mM) (Aa and Ab, right) in both capsaicin-insensitive (Aa) and capsaicin-sensitive (Ab) rat TG neurons. (B) The summary of the inhibition of ATP-induced P2X currents in both capsaicin-insensitive (Ba) and capsaicin-sensitive (Bb) rat TG neurons. The amplitude of second (C2) and third currents (C3) was normalized compared to the first one (C1). Eugenol-induced inhibition in capsaicin-insensitive neurons (n=15) was similar to that obtained in capsaicin-sensitive neurons (n=10) (mean±SEM, p>0.05). Black points indicate the time point of ATP application. |
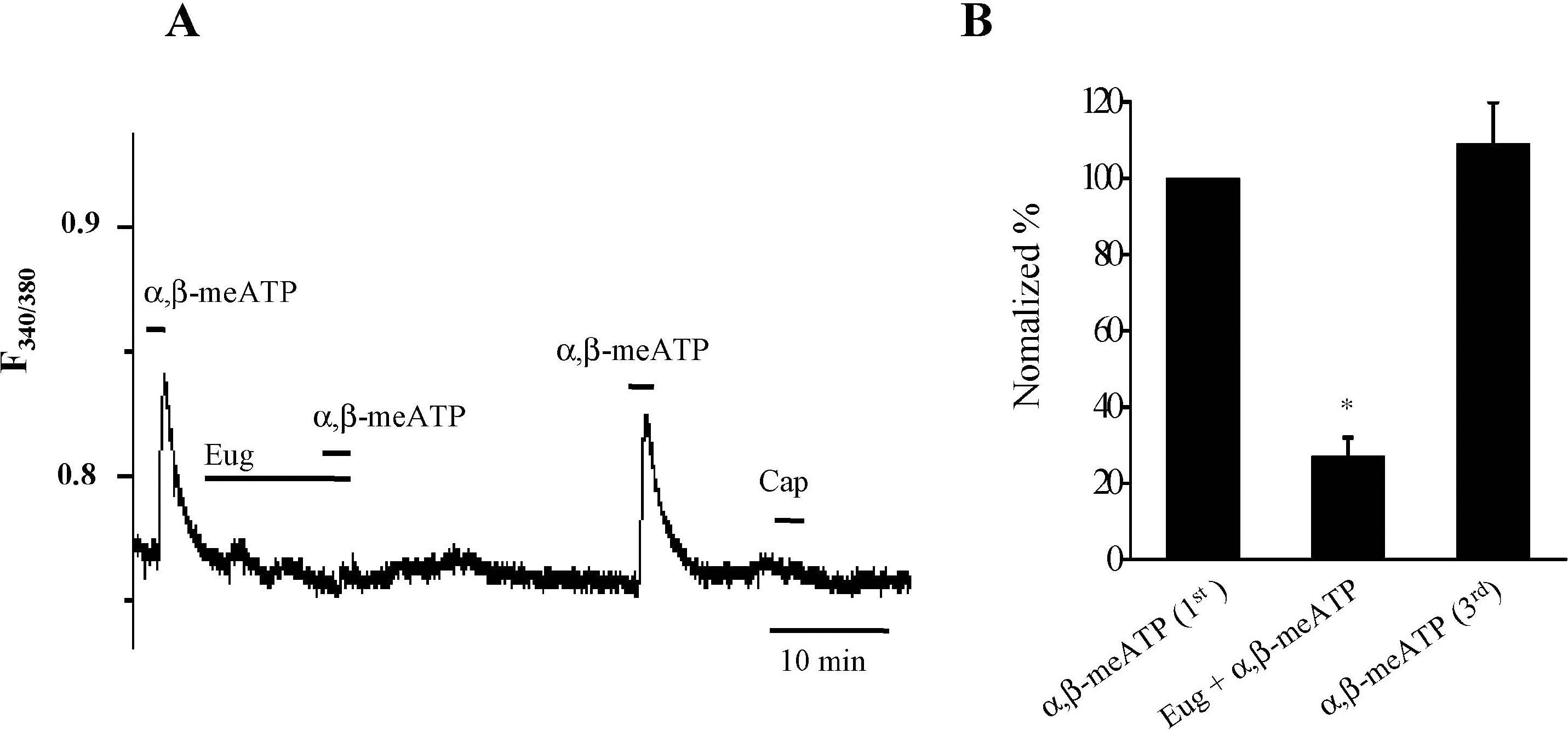 | Fig. 3.Eugenol inhibited α,β-meATP-induced Ca transients in rat TG neurons. (A) α,β-meATP (100 βM) induced Ca2+ transients in small-sized TG neurons. Eugenol (1 mM) abolished α,β-meATP-induced Ca2+ transients in capsaicin (0.5 μM)-insensitive TG neurons. (B) The summary of the inhibition of α,β-meATP-induced Ca2+ transients by eugenol in capsaicin-insensitive TG neurons (mean±SEM, n=9, p<0.05). The amplitude changes of Ca2+ transients induced by second (combined application with eugenol) and third (3rd) α,β-meATP applications were normalized compared to the first one (1st). |
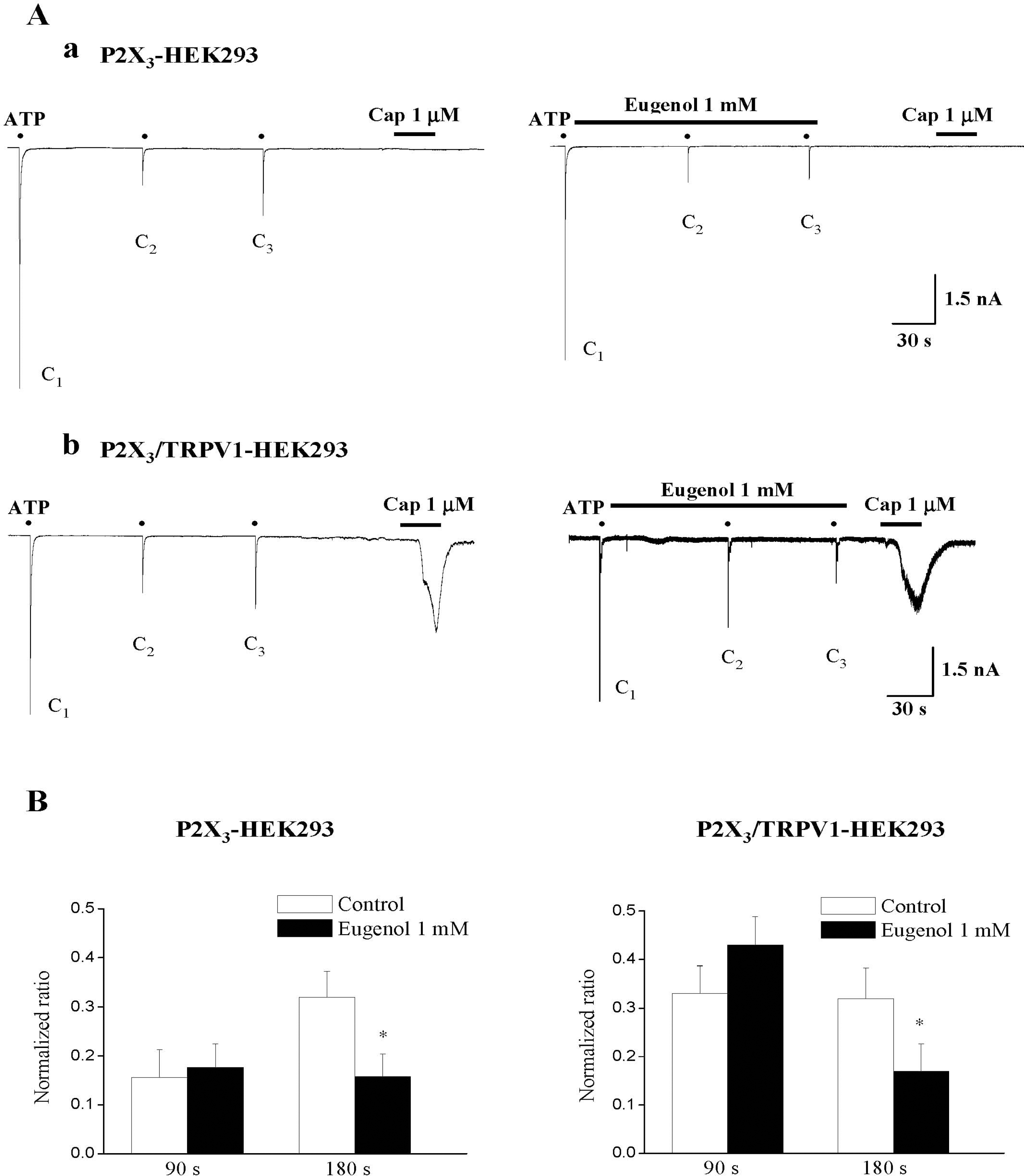 | Fig. 4.Eugenol inhibited ATP-induced P2X3 currents in both P2X3-expressing and P2X3/TRPV1-coexpressing HEK293 cells. (A) Representative current traces of 10 μM ATP-induced P2X3 currents under control (Aa and Ab, left), and eugenol (1 mM) (Aa and Ab, right) in both P2X3-expressing (Aa) and P2X3/TRPV1-coexpressing (Ab) HEK293 cells. (B) The summary of the inhibition of ATP-induced P2X3 currents in both P2X3-expressing (Aa) and P2X3/TRPV1-coexpressing (Ab) HEK293 cells. The amplitude of second (C2) and third currents (C3) was normalized compared to the first one (C1). Eugenol-induced inhibition in P2X3-expressing cells (n=13) was similar to that obtained in P2X3/TRPV1-coexpressing cells (n=12) (mean±SEM, p>0.05). Black points indicate the time point of ATP application. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download