Abstract
Sometimes, spinal cord injury (SCI) results in various chronic neuropathic pain syndromes that occur diffusely below the level of the injury. It has been reported that behavioral signs of neuropathic pain are expressed in the animal models of contusive SCI. However, the observation period is relatively short considering the natural course of pain in human SCI patients. Therefore, this study was undertaken to examine the time course of mechanical and cold allodynia in the hindpaw after a spinal cord contusion in rats for a long period of time (30 weeks). The hindpaw withdrawal threshold to mechanical stimulation was applied to the plantar surface of the hindpaw, and the withdrawal frequency to the application of acetone was measured before and after a spinal contusion. The spinal cord contusion was produced by dropping a 10 g weight from a 6.25 and 12.5 mm height using a NYU impactor. After the injury, rats showed a decreased withdrawal threshold to von Frey stimulation, indicating the development of mechanical allodynia which persisted for 30 weeks. The withdrawal threshold between the two experimental groups was similar. The response frequencies to acetone increased after the SCI, but they were developed slowly. Cold allodynia persisted for 30 weeks in 12.5 mm group. The sham animals did not show any significant behavioral changes. These results provide behavioral evidence to indicate that the below-level pain was well developed and maintained in the contusion model for a long time, suggesting a model suitable for pain research, especially in the late stage of SCI or for long term effects of analgesic intervention.
Go to : 
REFERENCES
Basso DM., Beattie MS., Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 12:1–21. 1995.

Basso DM., Beattie MS., Bresnahan JC. Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Exp Neurol. 139:244–256. 1996a.

Basso DM., Beattie MS., Bresnahan JC., Anderson DK., Faden AI., Gruner JA., Holford TR., Hsu CY., Noble LJ., Nockels R., Perot PL., Salzman SK., Young W. MASCIS evaluation of open field locomotor scores: effects of experience and teamwork on reliability. Multicenter Animal Spinal Cord Injury Study. J Neurotrauma. 13:343–359. 1996b.
Behrmann DL., Bresnahan JC., Beattie MS. Modeling of acute spinal cord injury in the rat: neuroprotection and enhanced recovery with methylprednisolone, U-74006F and YM-14673. Exp Neurol. 126:61–75. 1994.

Beric A., Dimitrijevic MR., Lindblom U. Central dysesthesia syndrome in spinal cord injury patients. Pain. 34:109–116. 1988.
Berrocal Y., Pearse DD., Singh A., Andrade CM., McBroom JS., Puentes R., Eaton MJ. Social and environmental enrichment improves sensory and motor recovery after severe contusive spinal cord injury in the rat. J Neurotrauma. 24:1761–1772. 2007.

Brewer KL., Yezierski RP. Effects of adrenal medullary transplants on pain-related behaviors following excitotoxic spinal cord injury. Brain Res. 798:83–92. 1998.

Bunge RP., Puckett WR., Hiester ED. Observations on the pathology of several types of human spinal cord injury, with emphasis on the astrocyte response to penetrating injuries. Adv Neurol. 72:305–315. 1997.
Chaplan SR., Bach FW., Pogrel JW., Chung JM., Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 53:55–63. 1994.

Choi Y., Yoon YW., Na HS., Kim SH., Chung JM. Behavioral signs of ongoing pain and cold allodynia in a rat model of neuropathic pain. Pain. 59:369–376. 1994.
Christensen MD., Everhart AW., Pickelman JT., Hulsebosch CE. Mechanical and thermal allodynia in chronic central pain following spinal cord injury. Pain. 68:97–107. 1996.

Constantini S., Young W. The effects of methylprednisolone and the ganglioside GM1 on acute spinal cord injury in rats. J Neurosurg. 80:97–111. 1994.

Cruz-Almeida Y., Martinez-Arizala A., Widerstrom-Noga EG. Chronicity of pain associated with spinal cord injury: A longitudinal analysis. J Rehabil Res Dev. 42:585–594. 2005.

Davidoff G., Roth EJ., Casey KL. Clinical characteristics of central (dysesthetic) pain in spinal cord injury patients. Casey KL, editor. ed,. Pain and central nervous system disease: The central pain syndromes. New York: Raven;p. p. 77–83. 1991.
Decosterd I., Woolf CJ. Spared nerve injury: an animal model of persistent peripheral neuropathic pain. Pain. 87:149–158. 2000.

Finnerup NB., Jensen TS. Spinal cord injury pain-mechanisms and treatment. Eur J Neurol. 11:73–82. 2004.
Gale K., Kerasidis H., Wrathall JR. Spinal cord contusion in the rat: behavioral analysis of functional neurologic impairment. Exp Neurol. 88:123–134. 1985.

Gruner JA. A monitored contusion model of spinal cord injury in the rat. J Neurotrauma. 9:123–126. 1992.

Hao JX., Xu XJ., Aldskogius H., Seiger A., Wiesenfeld-Hallin Z. Photochemically induced transient spinal ischemia induces behavioral hypersensitivity to mechanical and cold stimuli, but not to noxious-heat stimuli, in the rat. Exp Neurol. 118:187–194. 1992.

Hao JX., Yu W., Xu XJ., Wiesenfeld-Hallin Z. Capsaicin-sensitive afferents mediate chronic cold, but not mechanical, allodynialike behavior in spinally injured rats. Brain Res. 722:177–180. 1996.

Hook MA., Liu GT., Washburn SN., Ferguson AR., Bopp AC., Huie JR., Grau JW. The impact of morphine after a spinal cord injury. Behav Brain Res. 179:281–293. 2007.

Kim J., Yoon YW., Hong SK., Na HS. Cold and mechanical allodynia in both hindpaws and tail following thoracic spinal cord hemisection in rats: time courses and their correlates. Neurosci Lett. 343:200–204. 2003.

Lee KH., Suh-Kim H., Choi JS., Jeun SS., Kim EJ., Kim SS., Yoon DH., Lee BH. Human mesenchymal stem cell transplantation promotes functional recovery following acute spinal cord injury in rats. Acta Neurobiol Exp (Wars). 67:13–22. 2007.
Lee SH., Jung J., Kim J., Kim K., Hong SK., Yoon YW. Behavioral signs of pain in a rat spinal contusion model. Program No. 981.4. 2004. Abstract Viewer/Itinerary Planner. Washington, DC: Society for Neuroscience.
Lindsey AE., LoVerso RL., Tovar CA., Hill CE., Beattie MS., Bresnahan JC. An analysis of changes in sensory thresholds to mild tactile and cold stimuli after experimental spinal cord injury in the rat. Neurorehabil Neural Repair. 14:287–300. 2000.
Metz GA., Curt A., van de MH., Klusman I., Schwab ME., Dietz V. Validation of the weight-drop contusion model in rats: a comparative study of human spinal cord injury. J Neurotrauma. 17:1–17. 2000.

Ramer MS., Harper GP., Bradbury EJ. Progress in spinal cord research – a refined strategy for the International Spinal Research Trust. Spinal Cord. 38:449–472. 2000.

Rosenzweig ES., McDonald JW. Rodent models for treatment of spinal cord injury: research trends and progress toward useful repair. Curr Opin Neurol. 17:121–131. 2004.

Siddall PJ., McClelland JM., Rutkowski SB., Cousins MJ. A longitudinal study of the prevalence and characteristics of pain in the first 5 years following spinal cord injury. Pain. 103:249–257. 2003.

Stormer S., Gerner HJ., Gruninger W., Metzmacher K., Follinger S., Wienke C., Aldinger W., Walker N., Zimmermann M., Paeslack V. Chronic pain/dysaesthesiae in spinal cord injury patients: results of a multicentre study. Spinal Cord. 35:446–455. 1997.

Tasker RR., Loeser JD. Central Pain States. Loeser JD, editor. ed,. Bonica's Management of Pain. Philadelphia: Lippincott Williams Wilkins;p. p. 433–457. 2001.
Vierck CJ Jr. Siddall P, Yezierski RP. Pain following spinal cord injury: animal models and mechanistic studies. Pain. 89:1–5. 2000.
Voda J., Hama A., Sagen J. FK506 reduces the severity of cutaneous hypersensitivity in rats with a spinal cord contusion. Neurosci Res. 58:95–99. 2007.

Wasner G., Lee BB., Engel S., McLachlan E. Residual spinothalamic tract pathways predict development of central pain after spinal cord injury. Brain. 131:2387–2400. 2008.

Yezierski RP. Pain following spinal cord injury: the clinical problem and experimental studies. Pain. 68:185–194. 1996.

Yezierski RP., Liu S., Ruenes GL., Kajander KJ., Brewer KL. Excitotoxic spinal cord injury: behavioral and morphological characteristics of a central pain model. Pain. 75:141–155. 1998.

Go to : 
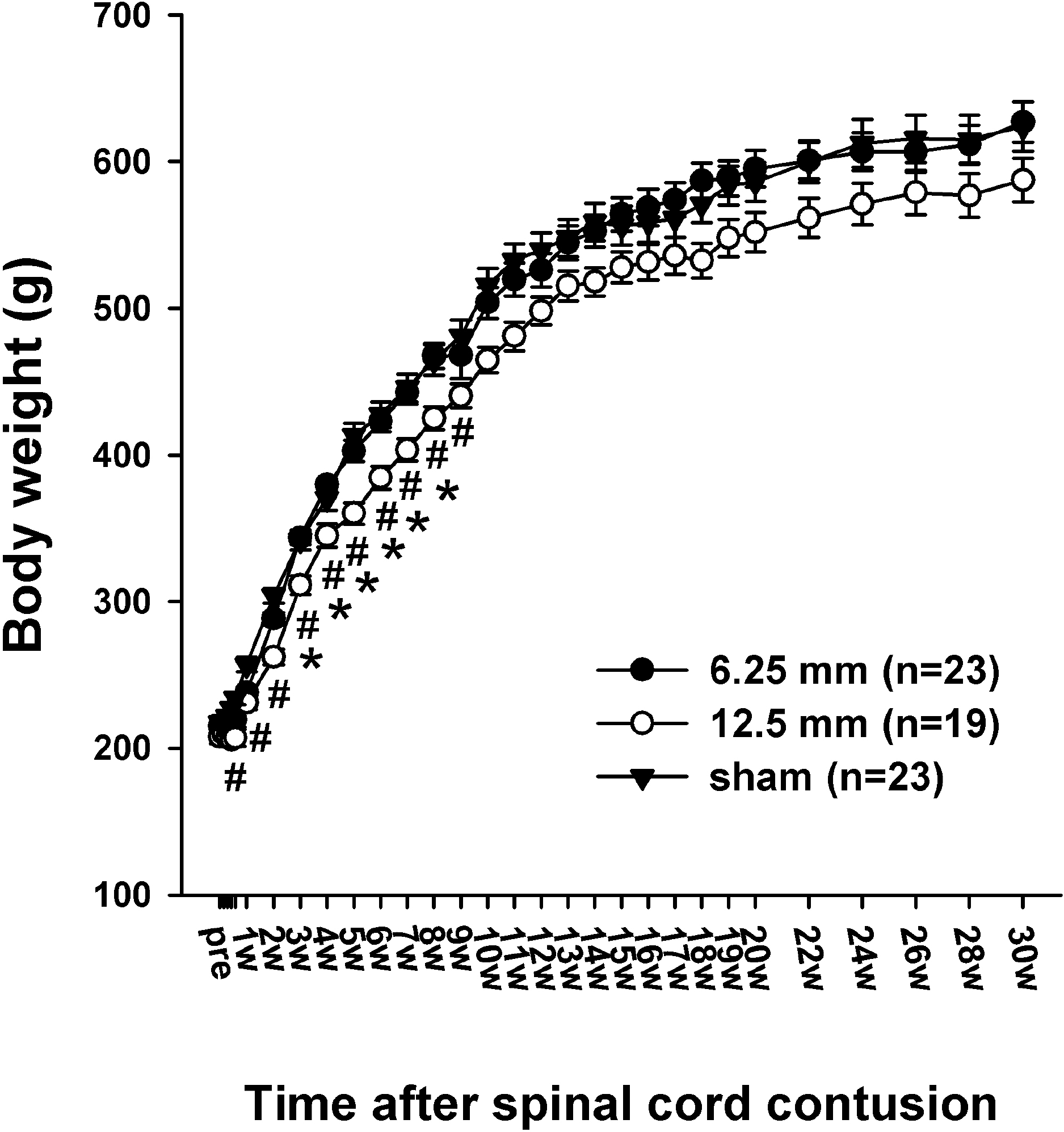 | Fig. 1.Time course of the body weight in the sham control (sham), 6.25 mm contusion, and 12.5 mm contusion groups. The average weight in all groups was approximately 220 g before the contusion. The rats in both the 6.25 and 12.5 mm groups showed an immediate reduction in body weight after the spinal contusion. Two weeks after the SCI, the weight of the rats in the 12.5 mm group (n=19) was significantly lower than that of the rats in the 6.25 mm group (n=23) or sham-operated group (n=23). There was no significant difference in weight between the sham group and the 6.25 mm group for the entire 30 weeks. The sharps (#) indicate the values significantly different between the sham and the 12.5 mm groups, and the asterisks (∗) indicate the values significantly different between the 6.25 mm group and 12.5 mm group. The time after the spinal cord contusion indicates weeks (W) following spinal contusion, except for “pre”, which represents the preoperative test period. |
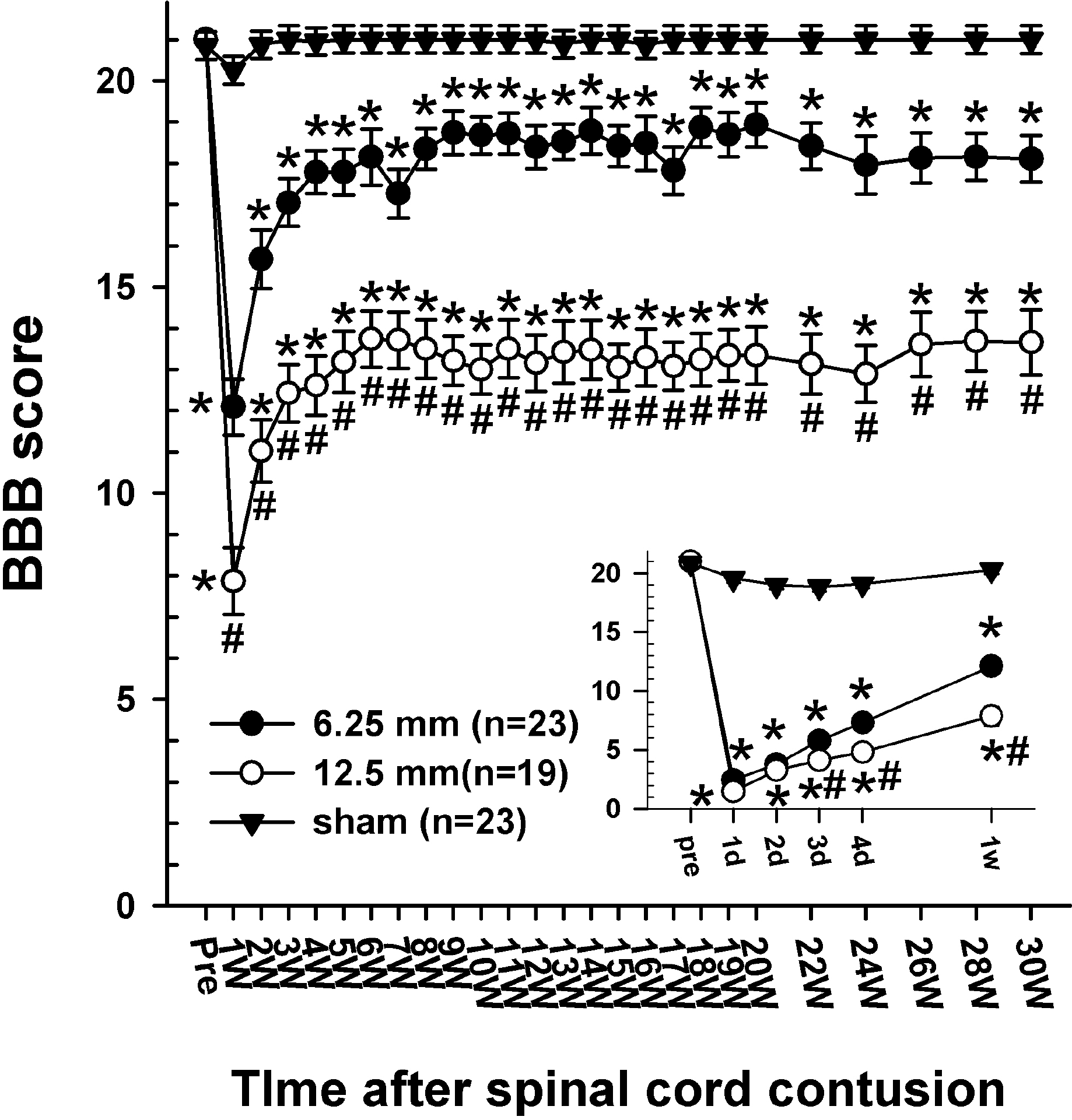 | Fig. 2.Time course of the BBB score following the spinal cord injury in three groups of rats. The inset shows the BBB score for the initial 7 days after spinal contusion for clarification. Before the SCI, the BBB score of all groups was 21. Every rat showed normal gaiting. On the first day after the SCI, the BBB scores decreased markedly to 2.4 ± 0.4 in the 6.25 mm groups and 1.5 ± 0.4 in the 12.5 mm group. By 3 weeks after the SCI, BBB scores gradually increased in both groups and were maintained for the remaining 27 weeks. Since 2 weeks after the SCI, the difference in the BBB score between the 6.25 mm group and the 12.5 mm group had obviously been sustained until 30 weeks after the SCI (p<0.05). The final BBB score of the 6.25 mm group was 18.1 ± 0.6 and that of the 12.5 mm group was 13.7 ± 0.8. The asterisks (∗) of each group indicate the values that are significantly different from the values in the sham group and sharps (#) indicate the values significantly different between the 6.25 mm group and the 12.5 mm group. The time after the spinal cord contusion indicates days (D) and weeks (W) following the spinal contusion, except for “pre”, which represents the preoperative test period. |
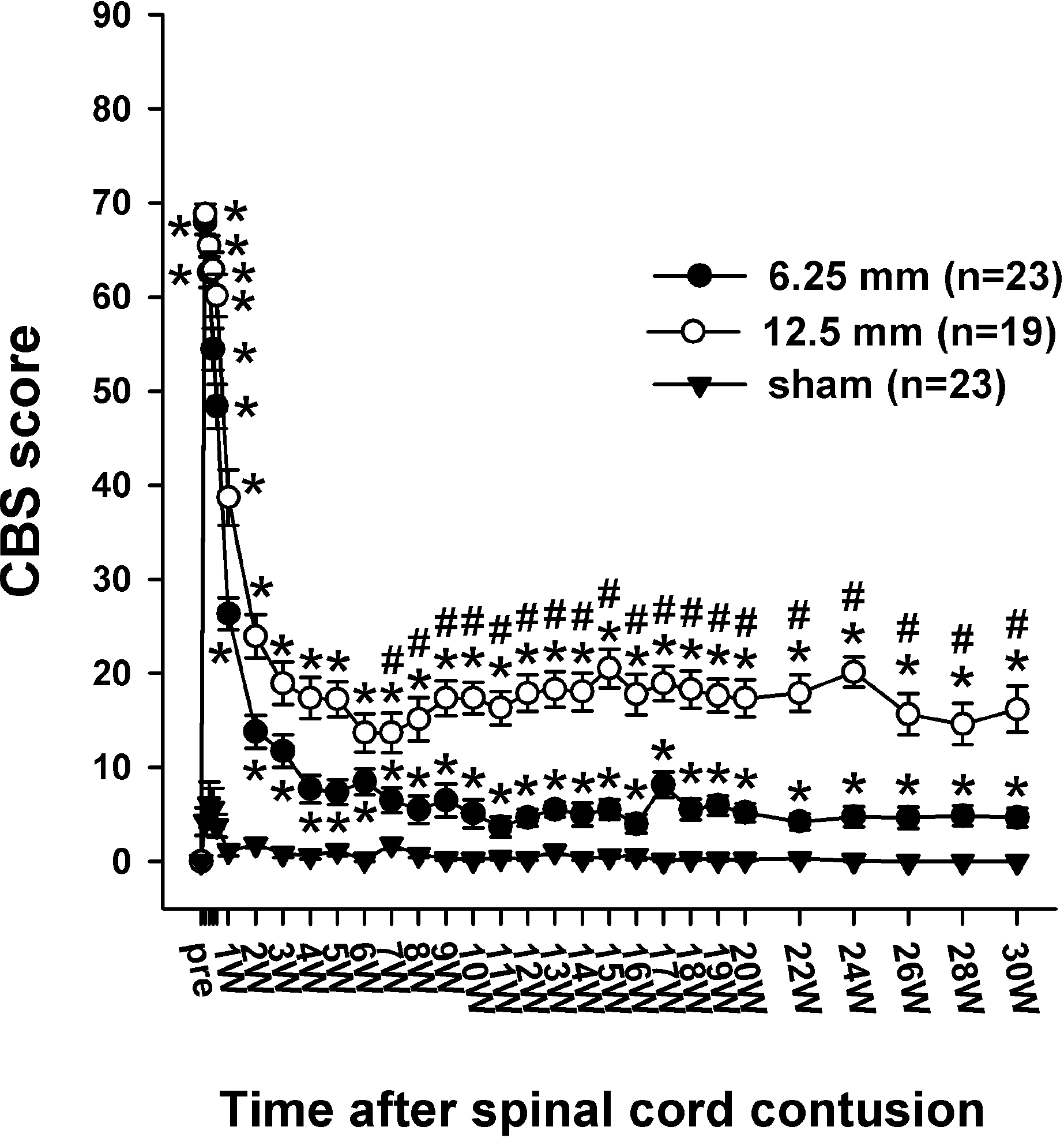 | Fig. 3.Time course of the CBS score (max.=90) following the spinal cord injury in the three groups. The CBS score of all the rats in pre-operation was 0 in the three groups. One day after the contusion injury, the CBS scores increased to 67.9 ± 1.3 in the 6. 25 mm group and 68.8 ± 1.1 in the 12.5 mm group. The CBS scores include the motor score, toe spread, righting reflex, extension withdrawal reflex, placing reflex and incline test. The asterisks (∗) of each group indicate the values significantly different from the sham group, and the sharps (#) I ndicate the values significantly different between the 6.25 mm group and 12.5 mm group. The time after the spinal cord contusion indicates weeks (W) following spinal contusion, except for “pre”, which represents the preoperative test period. |
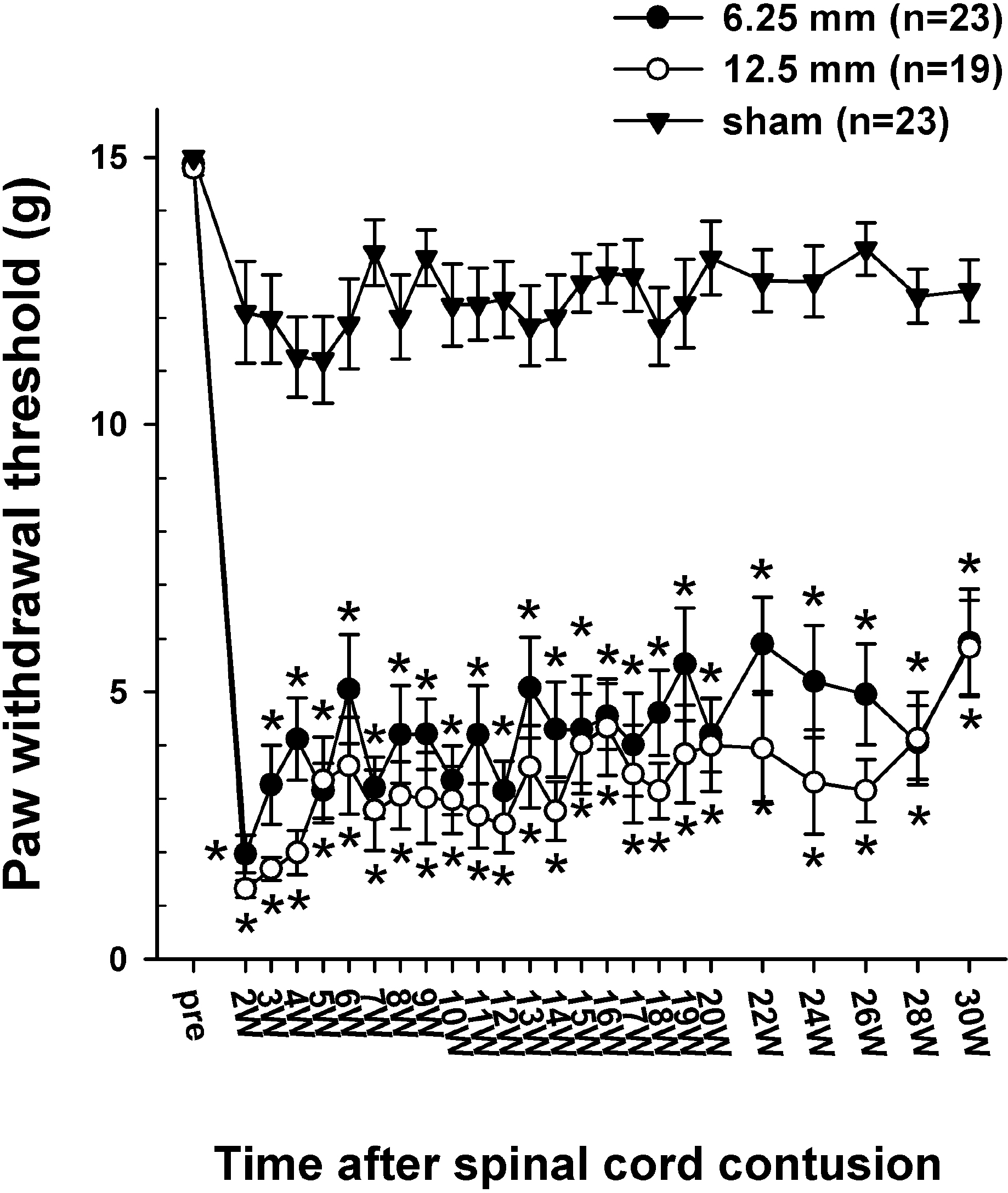 | Fig. 4.Time course of 50% withdrawal threshold to the graded von Frey filaments stimulation applied to the plantar surface of the hindpaw. The average paw withdrawal threshold before the contusion was approximately 15 g. However, following the contusion injury, the withdrawal thresholds to von Frey stimulation decreased markedly to 1.96 ± 0.35 g in the 6.25 mm group and 1.31 ± 0.16 g in the 12.5 mm group at 2 weeks PO and was slightly increased with the lapse of time. Asterisks (∗) indicate the values significantly different from the pre-operative value. The time after the spinal cord contusion indicates weeks (W) following spinal contusion, except for “pre”, which represents the preoperative test period. |
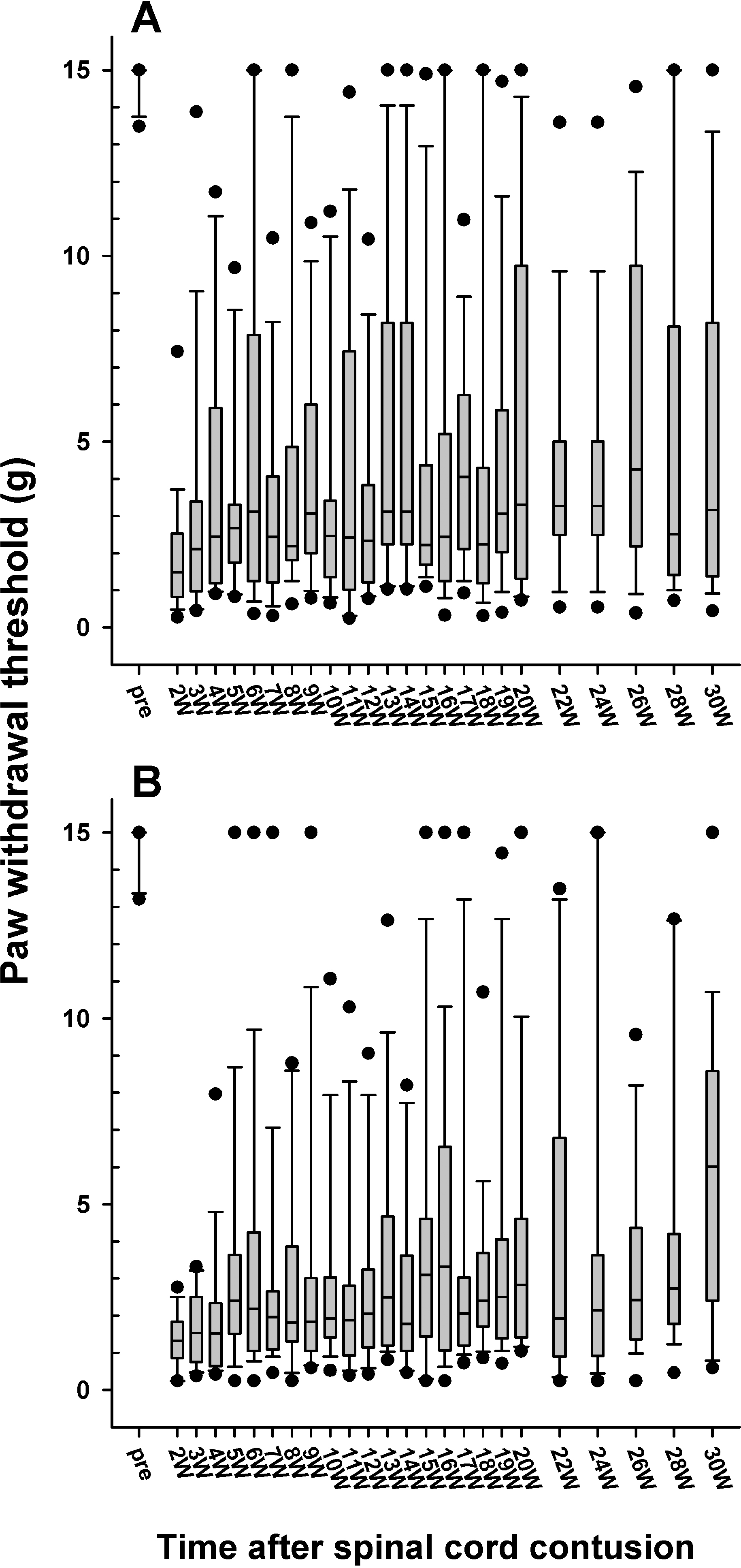 | Fig. 5.Follow-up of the paw withdrawal thresholds to mechanical stimulation in the 6.25 (A) and 12.5 mm (B) injury groups, which are expressed as a box plot. The data are expressed as a box plots with median (horizontal bar) and percentile points (25~75%; box) and 10~90% (error bar). The time after the spinal cord contusion indicates weeks (W) following the spinal contusion, except for “pre”, which represents the preoperative test period. |
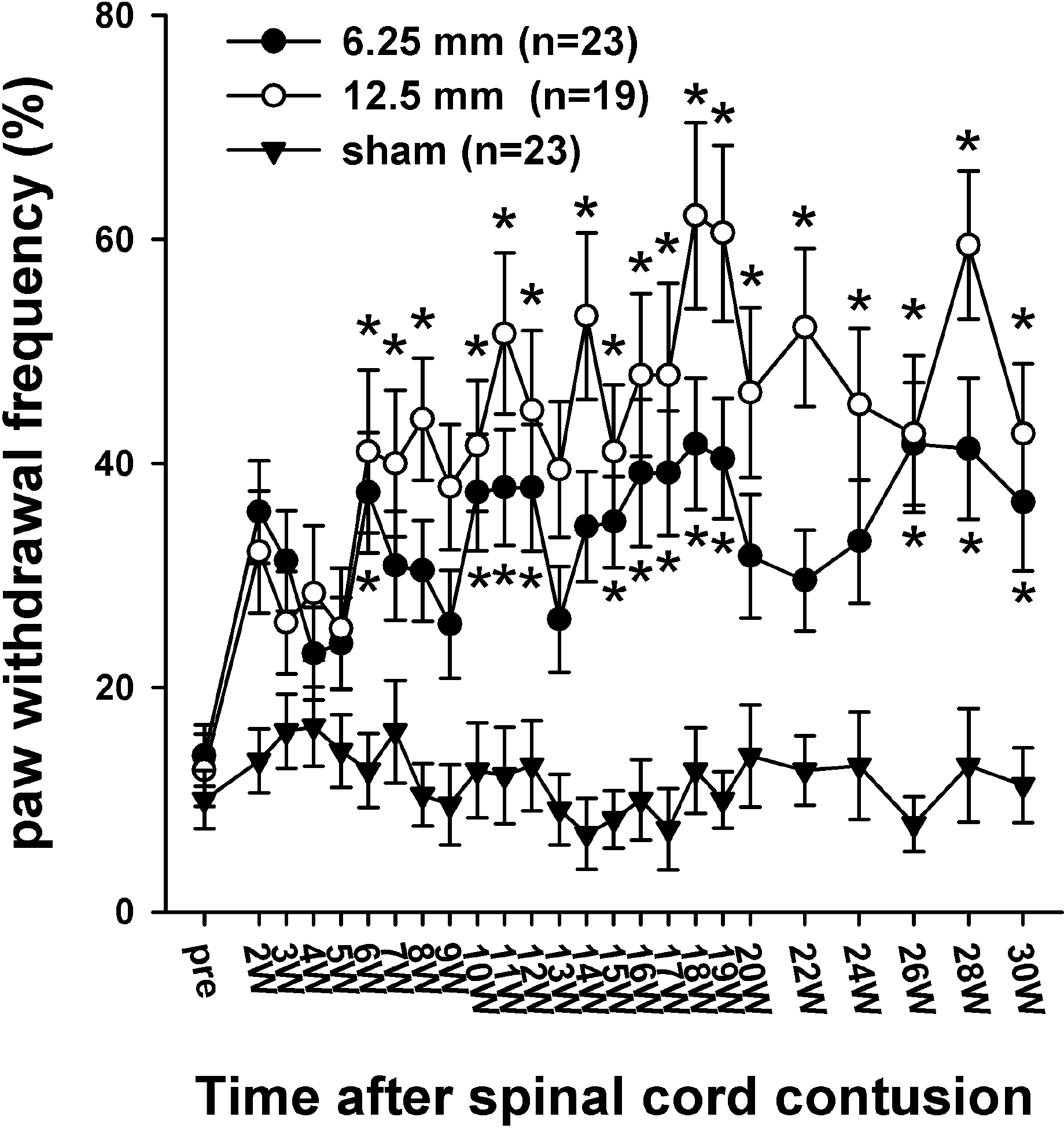 | Fig. 6.Time course of cold sensitivity. The frequencies of the foot withdrawals to cold stimuli caused by the application of acetone to the hindpaw are depicted. Before surgery, the withdrawal frequencies were 13.9 ± 2.7% in the 6.25 mm group and the 12.6 ± 3.2% in 12.5 mm group. Two weeks after the SCI, the withdrawal frequencies to the application of acetone in the 6.25- and 12.5 mm groups increased to 35.7 ± 4.6% and 32.1 ± 5.4%, respectively. The withdrawal responses in the 12.5 mm group were generally higher than those in the 6.25 mm, however, there was no difference between the two experimental groups. The asterisks (∗) indicate the values significantly different from the pre-operative value. The time after the spinal cord contusion indicates weeks (W) following the spinal contusion, except for “pre”, which represents the preoperative test period. |
Table 1.
Combined behavioral score (CBS) reported by Gale et al. (1985)




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download