Abstract
[Ca2+]i transients by reverse mode of cardiac Na+/Ca2+ exchanger (NCX1) were recorded in fura-2 loaded BHK cells with stable expression of NCX1. Repeated stimulation of reverse NCX1 produced a long-lasting decrease of Ca2+ transients (‘rundown’). Rundown of NCX1 was independent of membrane PIP2 depletion. Although the activation of protein kinase C (PKC) was observed during the Ca2+ transients, neither a selective PKC inhibitor (calphostin C) nor a PKC activator (PMA) changed the degrees of rundown. By comparison, a non-specific PKC inhibitor, staurosporine (STS), reversed rundown in a dose-dependent and reversible manner. The action of STS was unaffected by pretreatment of the cells with calphostin C, PMA, or forskolin. Taken together, the results suggest that the stimulation of reverse NCX1 by STS is independent of PKC and/or PKA inhibition.
Go to : 
REFERENCES
Blaustein MP., Lederer WJ. Sodium/calcium exchange: its physiological implications. Physiol Rev. 79:763–854. 1999.

Choi JS., Hahn SJ., Rhie DJ., Jo YH., Kim MS. Staurosporine directly blocks Kv1.3 channels expressed in Chinese hamster ovary cells. Naunyn-Schmiedebergs Arch. Pharmacol. 359:256–261. 1999.
Egger M., Porzig H., Niggli E., Schwaller B. Rapid turnover of the “functional” Na+-Ca2+ exchanger in cardiac myocytes revealed by an antisense oligodeoxynucleotide approach. Cell Calcium. 37:233–243. 2005.
Gescher A. Staurosporine analogues-pharmacological toys or useful antitumor agents? Crit Rev Oncol Hematol. 34:127–135. 2000.
Hilgemann DW. Regulation and deregulation of cardiac Na+-Ca2+ exchange in giant excised sarcolemmal membrane patches. Nature. 344:242–245. 1990.
Hilgemann DW., Ball R. Regulation of cardiac Na+,Ca2+ exchange and KATP potassium channels by PIP2. Science. 273:956–959. 1996.
Hilgemann DW., Collins A., Matsuoka S. Steady-state and dynamic properties of cardiac sodium-calcium exchange. Secondary modulation by cytoplasmic calcium and ATP. J Gen Physiol. 100:933–961. 1992a.

Hilgemann DW., Matsuoka S., Nagel GA., Collins A. Steady-state and dynamic properties of cardiac sodium-calcium exchange. Sodium-dependent inactivation. J Gen Physiol. 100:905–932. 1992b.

Iwamoto T., Pan Y., Nakamura TY., Wakabayashi S., Shigekawa M. Protein kinase C-dependent regulation of Na+/Ca2+ exchanger isoforms NCX1 and NCX3 does not require their direct phosphorylation. Biochemistry. 37:17230–17238. 1998.
Kang TM., Hilgemann DW. Multiple transport modes of the cardiac Na+/Ca2+ exchanger. Nature. 427:544–548. 2004.
Katanosaka Y., Iwata Y., Kobayashi Y., Shibasaki F., Wakabayashi S., Shigekawa M. Calcineurin inhibits Na+/Ca2+ exchange in phenylephrine-treated hypertrophic cardiomyocytes. J Biol Chem. 280:5764–5772. 2005.
Ko JH., Park WS., Earm YE. The protein kinase inhibitor, staurosporine, inhibits L-type Ca2+ current in rabbit atrial myocytes. Biochem Biophys Res Commun. 329:531–537. 2005.
Lee JE., Kang TM. Ca2+-dependent long-term inactivation of cardiac Na+/Ca2+ exchanger. Korean J Physiol Pharmacol. 11:183–188. 2007.
Linck B., Qiu Z., He Z., Tong Q., Hilgemann DW., Philipson KD. Functional comparison of the three isoforms of the Na+/Ca2+ exchanger (NCX1, NCX2, NCX3). Am J Physiol. 274:C415–423. 1998.
Opuni K., Reeves JP. Feedback inhibition of sodium/calcium exchange by mitochondrial calcium accumulation. J Biol Chem. 275:21549–21554. 2000.

Park WS., Son YK., Han J., Kim N., Ko JH., Bae YM., Earm YE. Staurosporine inhibits voltage-dependent K+ current through a PKC-independent mechanism in isolated coronary arterial smooth muscle cells. J Cardiovasc Pharmacol. 45:260–269. 2005.
Reeves JP., Condrescu M. Allosteric activation of sodium-calcium exchange activity by calcium: persistence at low calcium concentrations. J Gen Physiol. 122:621–639. 2003.
Reeves JP., Hale CC. The stoichiometry of the cardiac sodium-calcium exchange system. J Biol Chem. 259:7733–7739. 1984.

Ruëgg UT., Burgess GM. Staurosporine, K-252 and UCN-01: potent but nonspecific inhibitors of protein kinases. Trends Pharmacol Sci. 10:218–220. 1989.
Shen C., Lin MJ., Yaradanakul A., Lariccia V., Hill JA., Hilgemann DW. Dual control of cardiac Na+/Ca2+ exchange by PIP2: analysis of the surface membrane fraction by extracellular cysteine PEGylation. J Physiol. 582:1011–1026. 2007.
Yanagihara N., Tachikawa E., Izumi F., Yasugawa S., Yamamoto H., Miyamoto E. Staurosporine: an effective inhibitor for Ca2+/Calmodulin dependent protein kinase II. J Neurochem. 56:294–298. 1991.
Yang J., Liu X., Bhalla K., Kim CN., Ibrado AM., Cai J., Peng TI., Jones DP., Wang X. Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science. 275:1129–1132. 1997.

Yaradanakul A., Feng S., Shen C., Lariccia V., Lin MJ., Yang J., Kang TM., Dong P., Yin HL., Albanesi JP., Hilgemann DW. Dual control of cardiac Na+/Ca2+ exchange by PIP2: electrophysiological analysis of direct and indirect mechanisms. J Physiol. 582:991–1010. 2007.
Yaradanakul A., Wang TM., Lariccia V., Lin MJ., Shen C., Liu X., Hilgemann DW. Massive Ca-induced membrane fusion and phospholipid changes triggered by reverse Na/Ca exchange in BHK fibroblasts. J Gen Physiol. 132:29–50. 2008.

Zhang XQ., Ahlers BA., Tucker AL., Song J., Wang J., Moorman JR., Mounsey JP., Carl LL., Rothblum LI., Cheung JY. Phospholemman inhibition of the cardiac Na+/Ca2+ exchanger. Role of phosphorylation. J Biol Chem. 281:7784–7792. 2006.
Go to : 
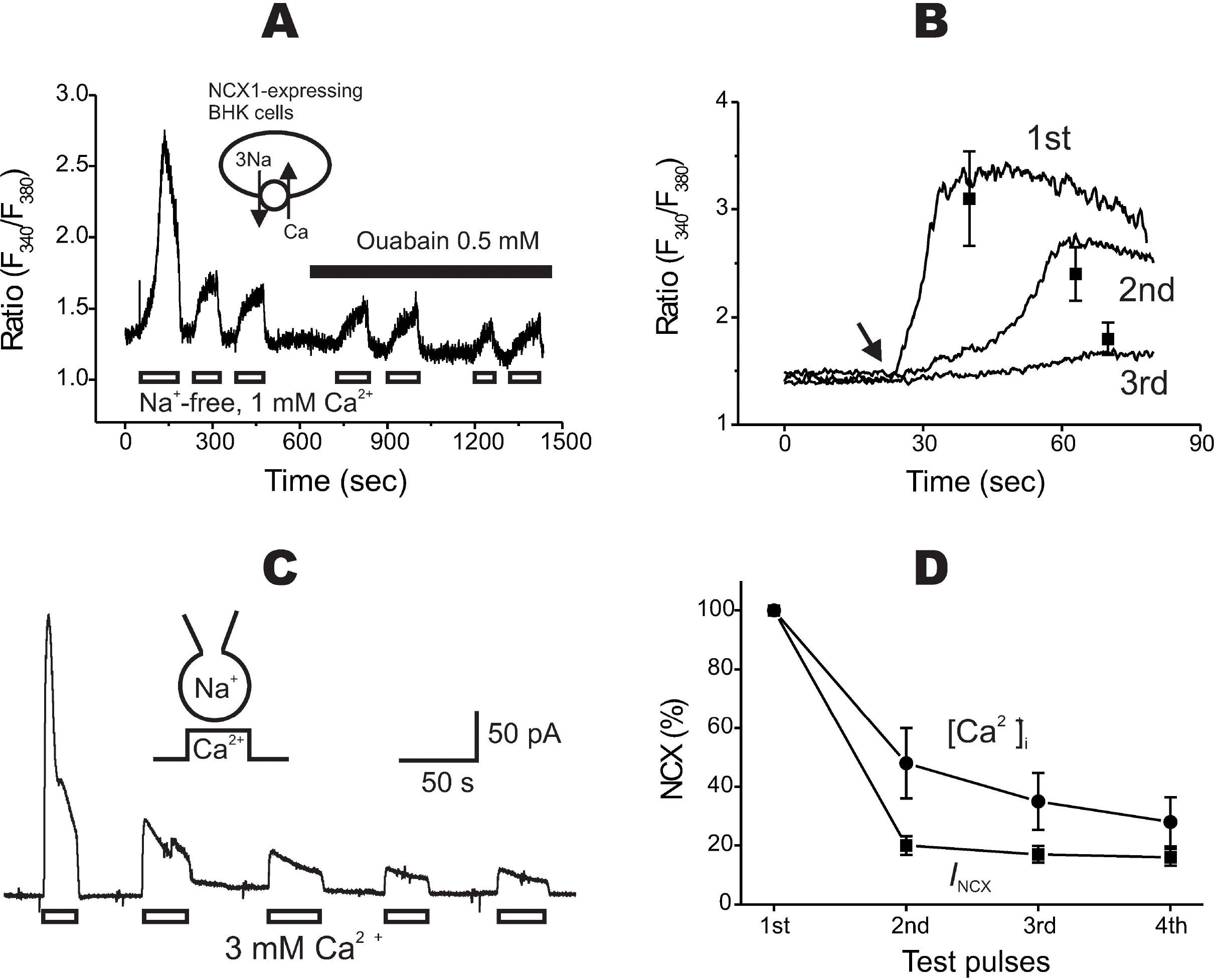 | Fig. 1.Rundown of reverse mode of Na+/Ca2+ exchanger (NCX1). (A) When NCX1 was repeatedly stimulated with Na+-free, 1 mM Ca2+-containing solution, a progressive decrease of the Ca2+ transients was observed. A Na+-pump inhibitor, ouabain, did not enhance the transients. (B) Increased rising time of the Ca2+ transients was observed during rundown. The representative traces recorded from the same cell were drawn for comparison. An arrow indicates the starting time of stimulation. The mean values of the time-to-peak values are described in text. The peak ratio values of the 1st, 2nd, and 3rd Ca2+ transient were 3.1±0.44, 2.4±0.25, and 1.8±0.15, respectively (n=4). (C) Rundown of the outward whole-cell NCX1 current. (D) The degrees and the time courses of rundown obtained from the Ca2+ transients (n=13) and the whole-cell current (n=7) were compared. Amplitudes of the 2nd, 3rd, and 4th Ca2+ transient were reduced to 48±12, 35±9.7, and 28±8.4% of the initial (‘1st’) transient, respectively. Amplitudes of the 2nd, 3rd, and 4th current were reduced to 20±3.2, 17±2.8, and 16±2.9% of the initial one, respectively. |
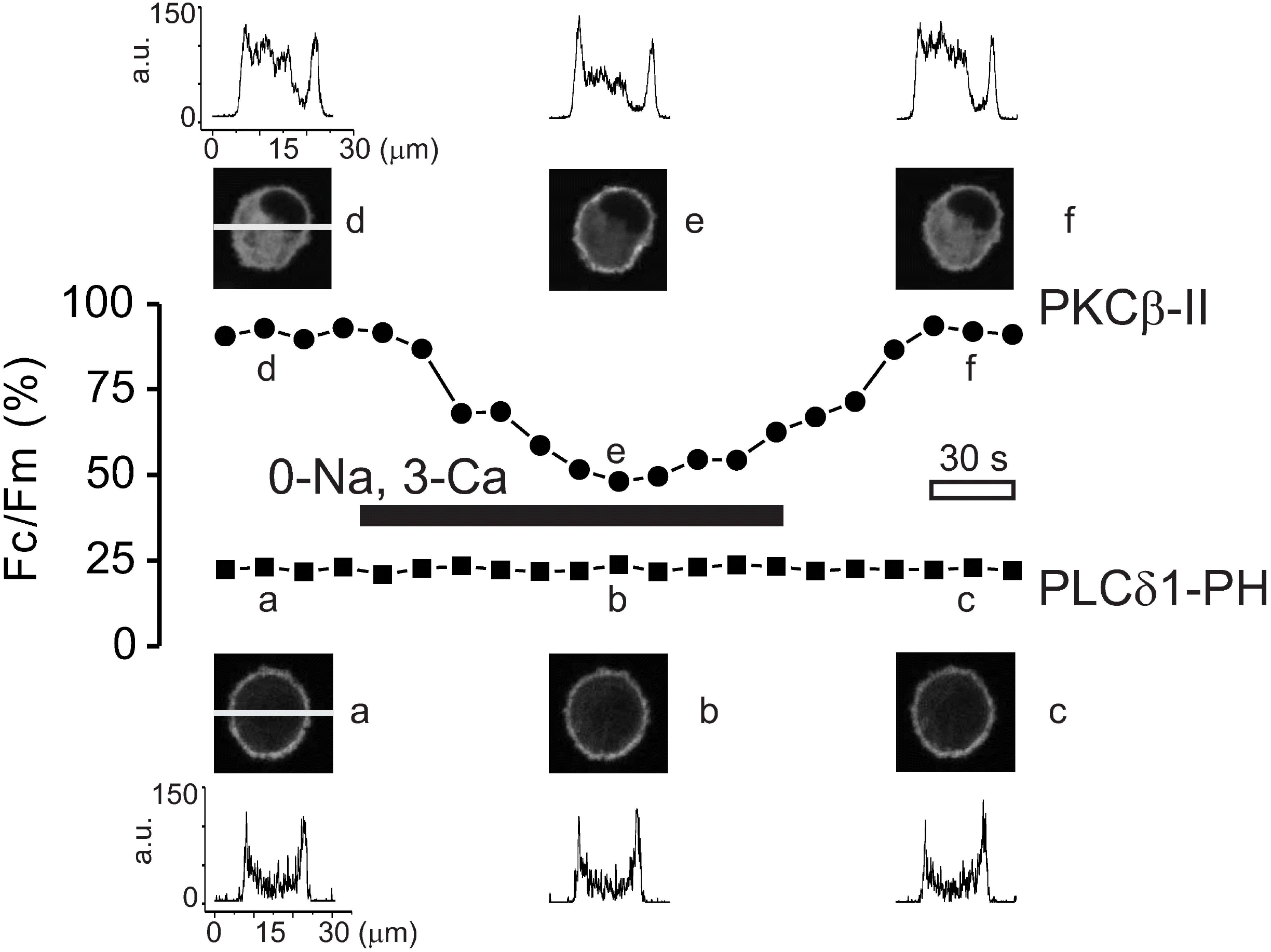 | Fig. 2.Using a confocal imaging, changes of the protein kinase C activity and the PIP2 concentration during the Ca2+ transients were monitored via PKCβ -II-GFP and PLCδ1-PH-GFP. Fluorescence intensity obtained along the horizontal axis was shown as histogram with corresponding confocal image. The normalized fluorescence intensity (Fc/Fm, %) was calculated at every 10 sec and plotted against time. Fc, fluorescence intensity of the cytosol; Fm, fluorescence intensity of the plasma membrane. (Upper panel) PKCβ-II-GFP, initially located in the cytosol, rapidly translocated to the plasma membrane upon Ca2+ influx via reverse NCX1. PKCβ-II-GFP returned to the cytosol when NCX1 was turned off. (Lower panel) No significant translocation of PH domain was monitored during the Ca2+ transient. |
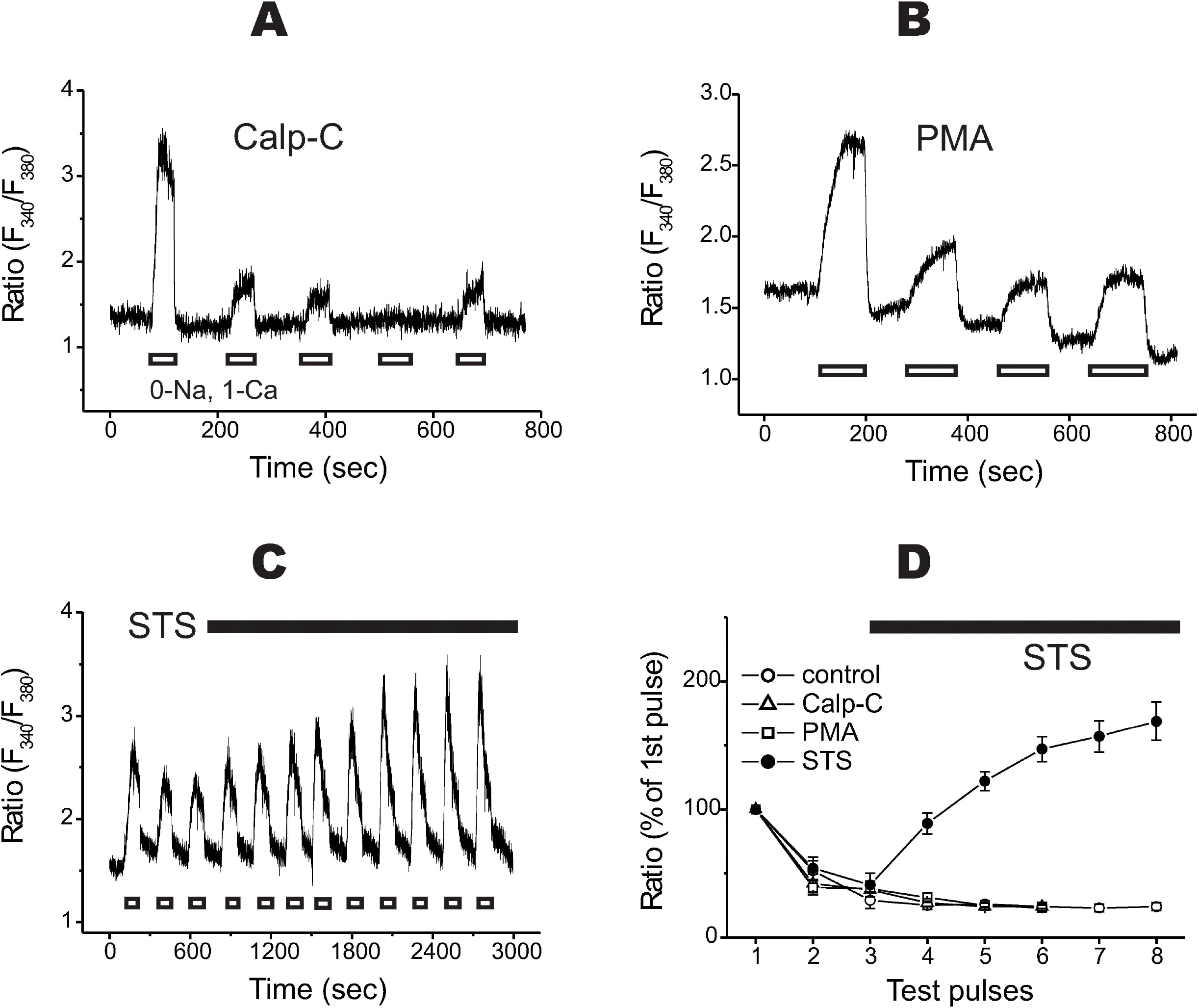 | Fig. 3.The effects of protein kinase C modulators on rundown. (A) Pretreatment of the cells with a selective PKC inhibitor, calphostin C (1μM), did not inhibit rundown of the Ca2+ transients. (B) Pretreatment of a strong PKC activator, PMA (0.1μ M), did not change rundown. (C) Normal rundown was recorded at the beginning of the recording (the first 3 repeats), and the 0.1μM STS was applied to the cells. The post-treatment of staurosporine (STS) recovered the Ca2+ transients and the final amplitudes were higher than the initial one. (D) Summarized data are illustrated. Control, rundown from control cells (n=8); Calp-C, pretreatment with calphostin C (n=6); PMA, pretreatment of phorbol ester (n=6); STS, post-treatment of STS after the 3rd transient (n=4). The black bars in C and D denote the period of time with STS. |
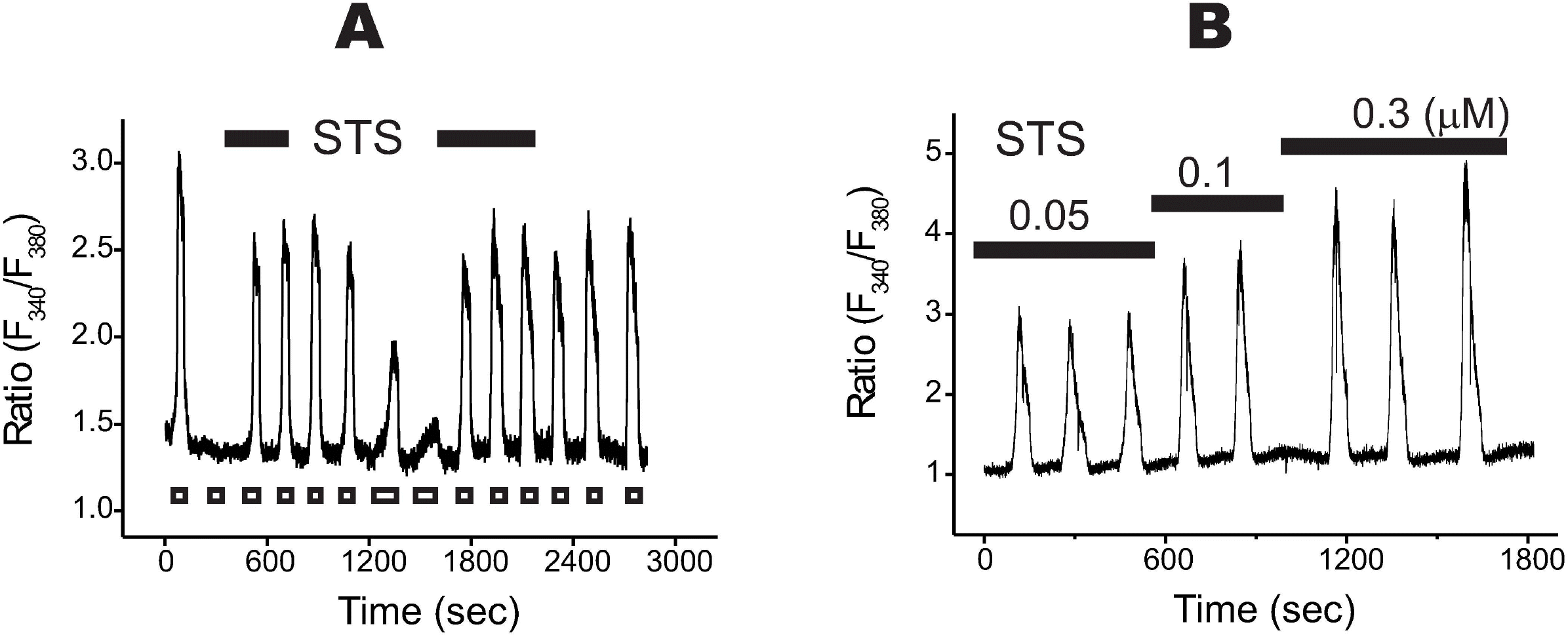 | Fig. 4.Staurosporine (STS) stimulated Na+/Ca2+ exchanger in a reversible and a dose-dependent manner. (A) In this recording, the initial Ca2+ transient was followed by a complete rundown of NCX1 (i.e. the 2nd transient was completely disappeared). When STS (0.1μM) was briefly applied prior to the 3rd one, it rapidly restored the Ca2+ transients. After the washing of STS, the next rundown was processed. By the second application of STS, restoration of the transients started again. (B) A lower concentration of STS (50 nM), that was applied before the initial stimulation, significantly inhibited rundown (compare the amplitudes of the initial 3 Ca2+ transients). When the concentrations of STS were increased up to 0.3μM, the amplitudes of the transients were increased dose-dependently. |
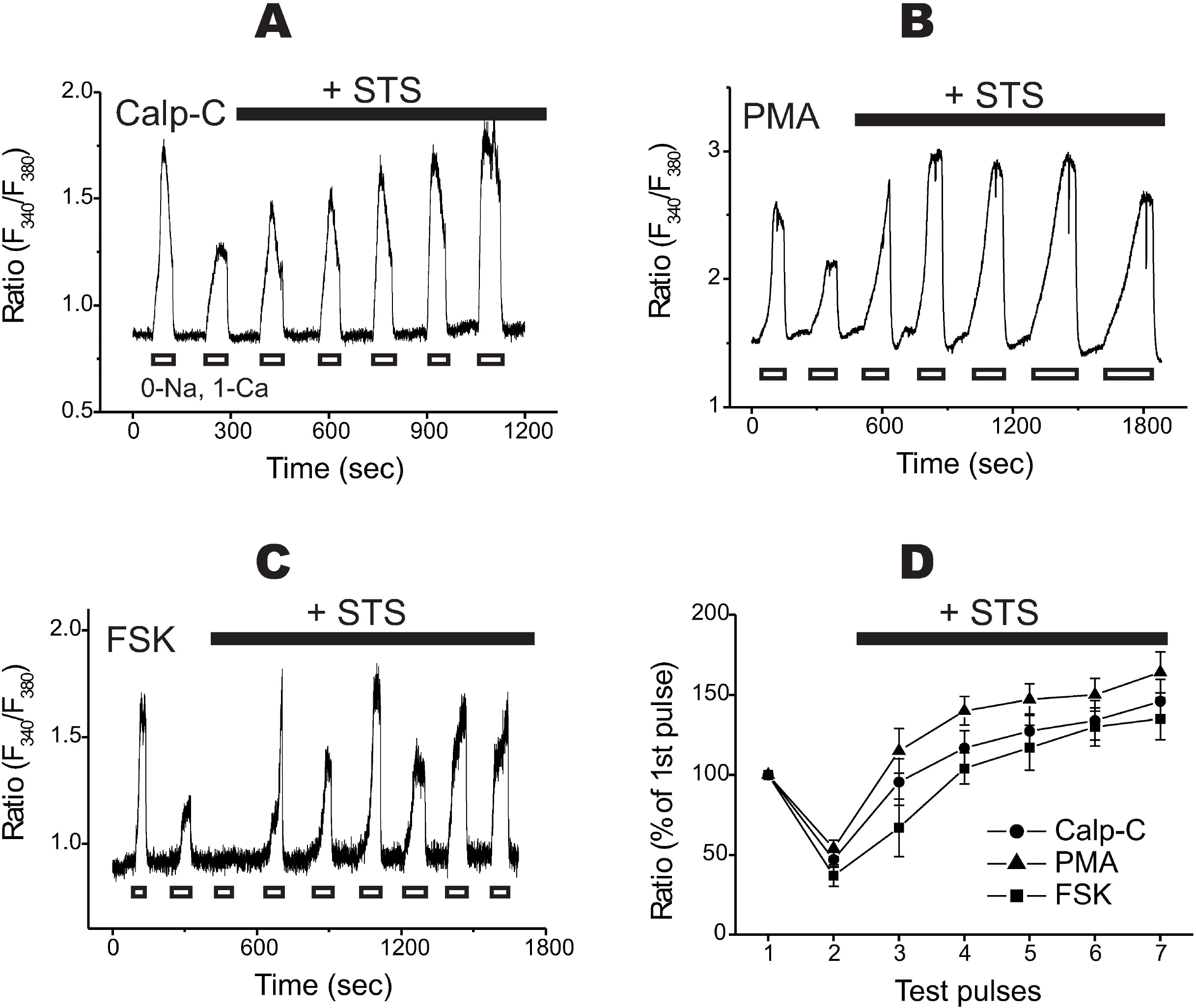 | Fig. 5.STS-increased Na+/Ca2+ exchanger is independent of protein kinase C and/or protein kinase A. (A~C) Even in the presence of calphostin C (1μM), PMA (0.1μM), and a PKA activator forskolin (FSK, 1μM), rundown of NCX1 was normally developed (i.e. the 2nd or 3rd transient was markedly decreased). However, the addition of STS (0.1μM) with each chemical almost completely restored the Ca2+ transients. STS was applied after the recording of the 2nd Ca2+ transient. (D) Summarized data were illustrated with time courses of rundown. Calp-C, calphostin C with STS (n=4); PMA, PMA with STS (n=7); FSK, forskolin with STS (n=5). |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download