Abstract
Somatostatin (SOM) is a widely distributed peptide in the central nervous system and exerts a variety of hormonal and neural actions. Although SOM is assumed to play an important role in spinal nociceptive processing, its exact function remains unclear. In fact, earlier pharmacological studies have provided results that support either a facilitatory or inhibitory role for SOM in nociception. In the current study, the effects of SOM were investigated using anesthetized cats. Specifically, the responses of rostrally projecting spinal dorsal horn neurons (RPSDH neurons) to different kinds of noxious stimuli (i.e., heat, mechanical and cold stimuli) and to the Aδ-and C-fiber activation of the sciatic nerve were studied. Iontophoretically applied SOM suppressed the responses of RPSDH neurons to noxious heat and mechanical stimuli as well as to C-fiber activation. Conversely, it enhanced these responses to noxious cold stimulus and Aδ-fiber activation. In addition, SOM suppressed glutamate-evoked activities of RPSDH neurons. The effects of SOM were blocked by the SOM receptor antagonist cyclo-SOM. These findings suggest that SOM has a dual effect on the activities of RPSDH neurons; that is, facilitation and inhibition, depending on the modality of pain signaled through them and its action site.
Go to : 
REFERENCES
Beitz AJ., Shepard RD., Wells WE. The periaqueductal gray- raphe magnus projection contains somatostatin, neurotensin and serotonin but not cholecystokinin. Brain Res. 261:132–137. 1983.
Carlton SM., Du J., Zhou S., Coggeshall RE. Tonic control of peripheral cutaneous nociceptors by somatostatin receptors. J Neurosci. 21:4042–4049. 2001.

Chapman V., Dickenson AH. The effects of sandostatin and somatostatin on nociceptive transmission in the dorsal horn of the rat spinal cord. Neuropeptides. 23:147–152. 1992.

Chrubasik J., Meynadier J., Scherpereel P., Wünsch E. The effect of epidural somatostatin on postoperative pain. Anesth Analg. 64:1085–1088. 1985.

Dichter MA., Wang HL., Reisine T. Electrophysiological effects of somatostatin-14 and somatostatin-28 on mammalian central nervous system neurons. Metabolism. 39:86–90. 1990.

Fruhstorfer H., Zenz M., Notle H., Hensel H. Dissociated loss of cold and warm sensibility during regional anesthesia. Pflügers Arch. 349:73–82. 1974.
Gray DB., Pilar GR., Ford MJ. Opiate and peptide inhibition of transmitter release in parasympathetic nerve terminals. J Neurosci. 9:1683–1692. 1989.

Hanesch U., Heppelmann B., Schmidt RF. Somatostatin-like immuno-reactivity in primary afferents of the medial articular nerve and colocalization with substance P in the cat. J Comp Neurol. 354:345–352. 1995.

Helmchen C., Fu Q-C., Sandkühler J. Inhibition of spinal nociceptive neurons by microinjections of somatostatin into the nucleus raphe magnus and midbrain periaqueductal gray of the anesthetized cat. Neurosci Lett. 187:137–141. 1995.
Helyes Z., Thán M., Oroszi G., Pintér E., Németh J., Kéri G., Szolcsányi J. Anti-nociceptive effect induced by somatostatin released from sensory nerve terminals and by synthetic somatostatin analogues in the rat. Neurosci Lett. 278:185–188. 2000.

Hunt SP., Kelly JS., Emson PC., Kimmel JR., Miller RJ., Wu JY. An immunohitochemical study of neuronal populations containing neuropeptides or GABA within the superficial layers of the rat dorsal horn. Neurosci. 6:1883–1898. 1981.
Jiang N., Furue H., Katafuchi T., Yoshimura M. Somatostatin directly inhibits substantia gelatinosa neurons in adult rat spinal dorsal horn in vitro. Neurosci Res. 47:97–107. 2003.

Kamei J., Hitosugi H., Kasuya Y. Nociceptive responses to intrathecally administered substance P and somatostatin in diabetic mice. Life Sci. 52::p. L31–36. 1993.

Kamei J., Hitosugi H., Misawa M., Nagase H., Kasuya Y. Cold water swim stress inhibits the nociceptive responses to intrathecally administered somatostatin, but not substance P. Life Sci. 52:p. L169–174. 1993.

Kim SJ., Chung WH., Rhim H., Eun SY., Jung J., Kim J. Postsynaptic action mechanism of somatostatin on the membrane excitability in spinal substantia gelatinosa neurons of juvenile rats. Neurosci. 114:1139–1148. 2002.

Kleuss C., Hescheler J., Ewel C., Rosenthal W., Schultz G., Wittig R. Assignment of G-protein subtypes to specific receptors inducing inhibition of calcium currents. Nature. 353:43–48. 1991.

Kuraishi Y., Hirota N., Sato Y., Hino Y., Satoh M., Takagi H. Evidence that substance P and somatostatin transmit separate information related to pain in the dorsal horn. Brain Res. 325:294–298. 1985.
Lu J., Ho RH. Evidence for dorsal root projection to somatostatin-immunoreactive structures in laminae I-II of the spinal dorsal horn. Brain Res Bull. 28:17–26. 1992.

Mather CS., Ho RH. Golgi impregnated somatostatin immunore-active neurons in lamina II of the rat spinal cord. Brain Res Bull. 28:305–309. 1992.

Meynadier J., Chrubasik J., Dubar M., Wünsch E. Intrathecal somatostatin in terminally ill patients. A report of two cases. Pain. 23:9–12. 1985.

Millhorn DE., Seroogy K., Hökfelt T., Schmued LC., Terenius L., Buchan A., Brown JC. Neurons of the ventral medulla oblongata that contain both somatostatin and enkephalin immunoreactivities project to nucleus tractus solitarii and spinal cord. Brain Res. 424:99–108. 1987.

Mollenholt P., Post C., Rawal N., Freedman J., Hökfelt T., Paulsson I. Antinociceptive and “neurotoxic” actions of somatostatin in rat spinal cord after intrathecal administration. Pain. 32:95–105. 1988.

Morton CR., Hutchison WD., Hendry IA., Duggan AW. Somatostatin: evidence for a role in thermal nociception. Brain Res. 488:89–96. 1989.

Murase K., Nedeljkov V., Randic M. The actions of neuropeptides on dorsal horn neurons in the rat spinal cord slice preparation: an intracellular study. Brain Res. 234:170–176. 1982.

Ohno HY., Kuraishi M., Minami ., Satoh M. Modality-specific antinociception produced by intrathecal injection of anti-somatostatin antiserum in rats. Brain Res. 474:197–200. 1988.

Paice JA., Penn RD., Kroin JS. Intrathecal octreotide for relief of intractable nonmalignant pain: 5-year experience with two cases. Neurosurgery. 38:203–207. 1996.

Penn RD., Paice JA., Kroin JS. Octreotide: a potent new-non-opiate analgesic for intrathecal infusion. Pain. 49:13–19. 1992.
Pintér E., Helyes Z., Németh J., Pórszász R., Pethö G., Thán M., Kéri G., Horváth A., Jakab B., Szolcsányi J. Pharmacological characterisation of the somatostatin analogue TT-232: effects on neurogenic and non-neurogenic inflammation and neuropathic hyperalgesia. Naunyn Schmiedebergs Arch Pharmacol. 366:142–150. 2002.

Rang HP., Bevan S., Dray A. Nociceptive peripheral neurons: cellular properties. Wall PD, Melzack R, editors. eds,. Textbook of Pain. 3rd ed.Churchill Livingstone;Edinburgh (UK): p. p. 57–78. 1994.
Robbins R. Somatostatin and the cerebral cortex. Patel YC, Tanneum GS, editors. eds,. Somatostatin. Plenum;New York: p. p. 201–216. 1985.

Sandkühler J., Fu Q-G., Helmchen C. Spinal somatostatin superfusion in vivo affects activity of cat nociceptive dorsal horn neurones: Comparison of spinal morphine. Neurosci. 34:565–576. 1990.
Sandle GI., Warhurst G., Butterfield I., Higgs NB., Lomax RB. Somatostatin peptides inhibit basolateral potassium channels in human colonic crypts. Am J Physiol. 277:G967–975. 1999.
Seybold VS., Hylden JLK., Wilcox GL. Intrathecal substance P and somatostatin in rats: Behaviors indicative of sensation. Peptides. 3:49–54. 1982.

Sicuteri F., Geppetti P., Marabini S., Lembeck F. Pain relief by somatostatin in attacks of cluster headache. Pain. 18:359–365. 1984.

Simone DA., Kajander KC. Responses of cutaneous A-fiber nociceptors to noxious cold. J. Neurophysiol. 77:2049–2060. 1997.
Song P., Hu JY., Zhao ZQ. Spinal somatostatin SSTR2A receptors are preferentially up-regulated and involved in thermonociception but not mechanonociception. Exp Neurol. 178:280–287. 2002.

Stine SM., Yang HY., Costa E. Evidence for ascending and descending intraspinal as well as primary sensory somatostatin projections in the rat spinal cord. J Neurochem. 38:1144–1150. 1982.

Taddese A., Nah SY., McClesky EW. Selective opioid inhibition of small nociceptive neurons. Science. 270:1366–1369. 1995.

Traub RJ., Brozoski D. Anti-somatostatin antisera, but neither a somatostatin agonist (octreotide) nor antagonist (CYCAM), attenuates hyperalgesia in the rat. Peptides. 17:769–773. 1996.

Tsai YC., So EC., Chen HH., Wang LK., Chien CH. Effect of intra-thecal octreotide on thermal hyperalgesia and evoked spinal c-Fos expression in rats with sciatic constriction injury. Pain. 99:407–413. 2002.

Tuchscherer MM., Seybold VS. Immunohistochemical studies of substance P, cholecystokinin-octapeptide and somatostatin in dorsal root ganglia of the rat. Neurosci. 14:593–605. 1985.

Wang H., Bogen C., Reisine T., Dichter M. SRIF-14 and SRIF-28 induce opposite effects on potassium currents in rat neocortical neurons. Proc Natl Acad Sci USA. 86:9616–9620. 1989.
Wiesenfeld-Hallin Z. Intrathecal somatostatin modulates spinal sensory and reflex mechanisms: behavioral and electrophysiological studies in the rat. Neurosci Lett. 62:69–74. 1985.

Wiesenfeld-Hallin Z. Substance P and somatostatin modulate spinal cord excitability via physiologically different sensory pathways. Brain Res. 372:172–175. 1986.

Willis WD., Coggeshall RE. The sensory channel. Willis WD, Coggeshall RE, editors. eds,. Sensory Mechanisms of the Spinal Cord. 2nd ed.Plenum Press;New York: p. p. 449–456. 1991.
Go to : 
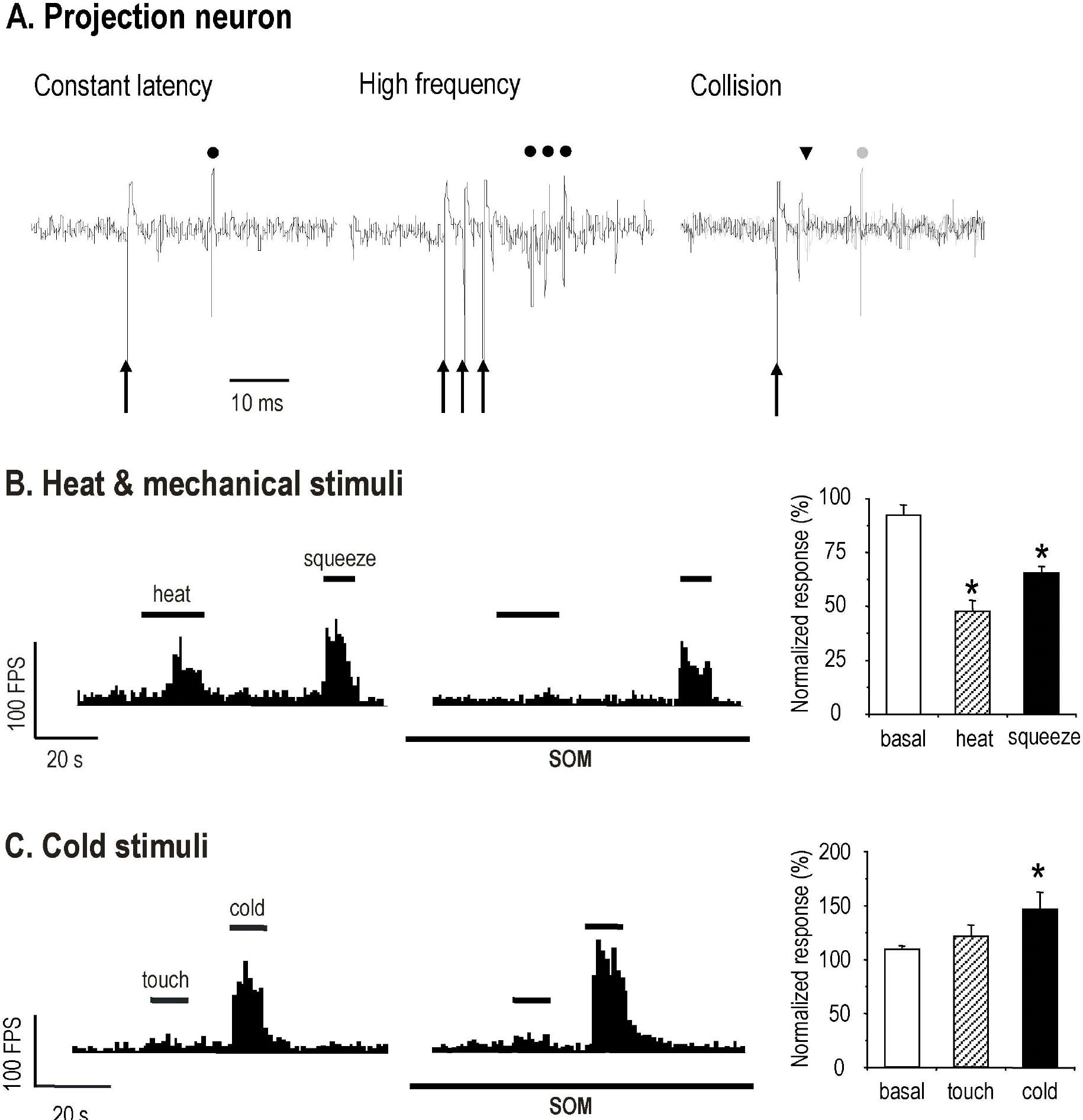 | Fig. 1.Effects of iontophoretically applied SOM on the RPSDH neuron response to peripheral noxious stimuli. Single cell activity was recorded in the lumbosacral area using an extracellular electrode. (A) RPSDH neurons exhibit several characteristics; namely, 1) constant latency, 2) the responses to high frequency (333 Hz) stimulation, and 3) collision. Arrow (↑) represents the electrical stimuli to sciatic nerve. The activity of RPSDH neuron (•) by electrical stimuli was observed after a constant latency. Gray circle (•) means the activity of RPSDH neuron, which vanished after collision (▼) by cervical dorsal column stimulation. In this experiment, RPSDH neurons were used. (B) Iontophoretic application of SOM (100 nA) resulted in the inhibition of nociceptive response to noxious heat (50°C) stimuli subjected for a 20 sec duration and to mechanical stimuli (squeeze) for 10 sec. After an iontophoretic application of SOM (100 nA), the heat-evoked and the noxious mechanically evoked responses were suppressed. (C) The effects of SOM on the response of RPSDH neuron to peripheral noxious cold stimulation are presented. SOM (100 nA) increased the cold-evoked response of the RPSDH neuron. Each bar graph represents mean value±standard error for SOM effect on noxious stimuli such as heat, squeeze, and cold. The asterisk shows significant difference in SOM effect (non paired t-test, p<0.05). |
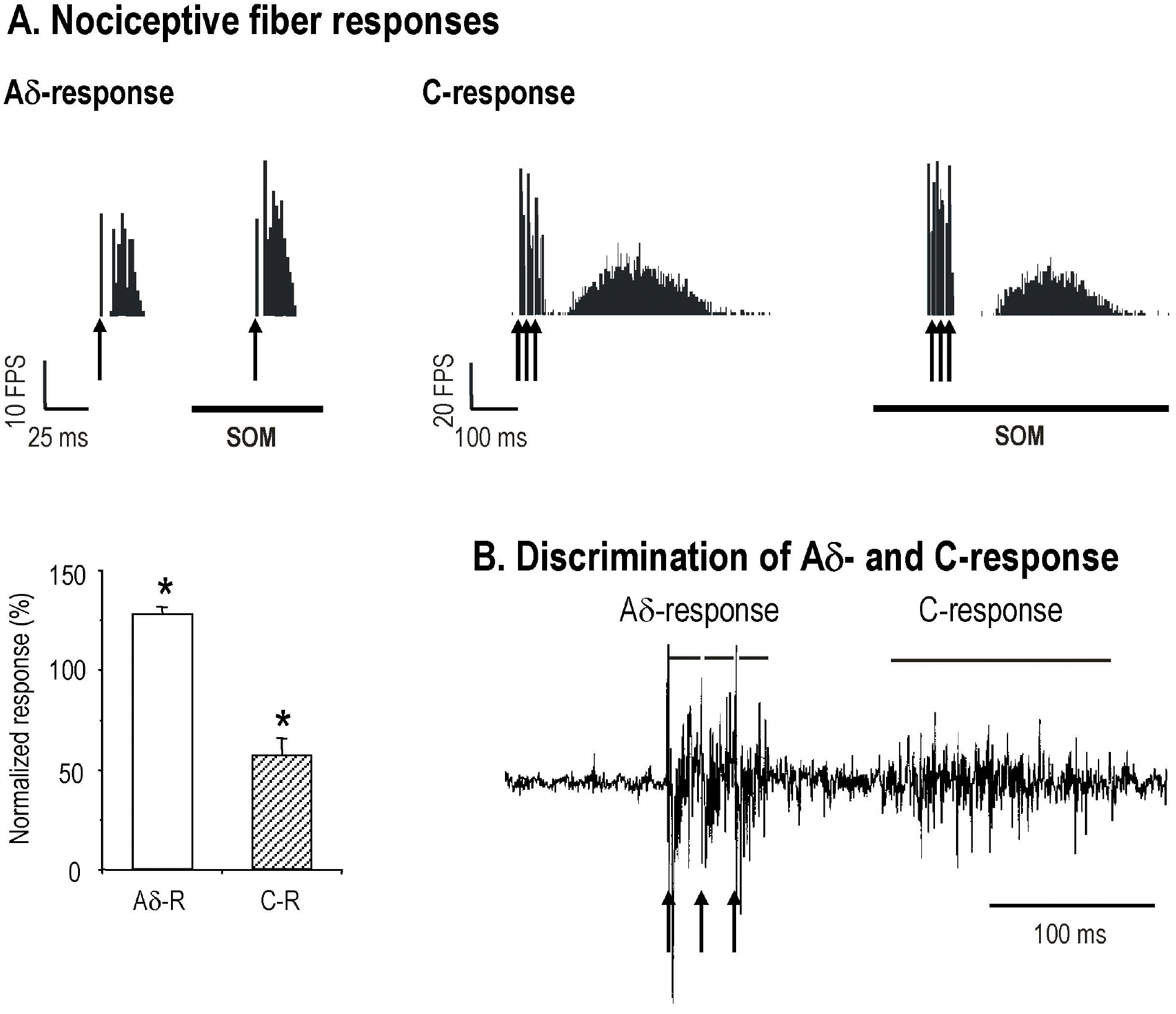 | Fig. 2.Effects of iontophoretically applied SOM on the RPSDH neuron response to noxious electrical stimuli of the peripheral nerve. (A) The single (↑) or triple (↑ ↑↑) electrical stimulation at 500 ms was applied to the sciatic nerve with Aδ-strength (1 mA with 0.1 ms width) or with C-strength (10 mA with 0.5 ms width). SOM increased Aδ -fiber response of this cell, whereas the C-fiber response of the same cell was markedly suppressed. The summary bar graph was derived from the normalized SOM effect on Aδ- (Aδ-R) and C-responses (C-R). (B) Electrical stimuli were applied to the sciatic nerve for the activation of Aδ-or C-fibers (a single or a train of three square wave pulses). In doing so, the responses were discriminated using window discriminator. The Aδ -response was the sum of activities appearing in less than 50 ms, while the C-response are those after 150 ms. Evoked responses were expressed as the total number of impulses. Also, twenty sweeps were compiled as a peristimulus time histogram (bin width; 2 ms, 20 sweeps). The bar graph represents mean value±standard error for SOM effect on noxious electrical stimuli. The significant difference in SOM effect (non paired t-test, p<0.05) is represented by the asterisk. |
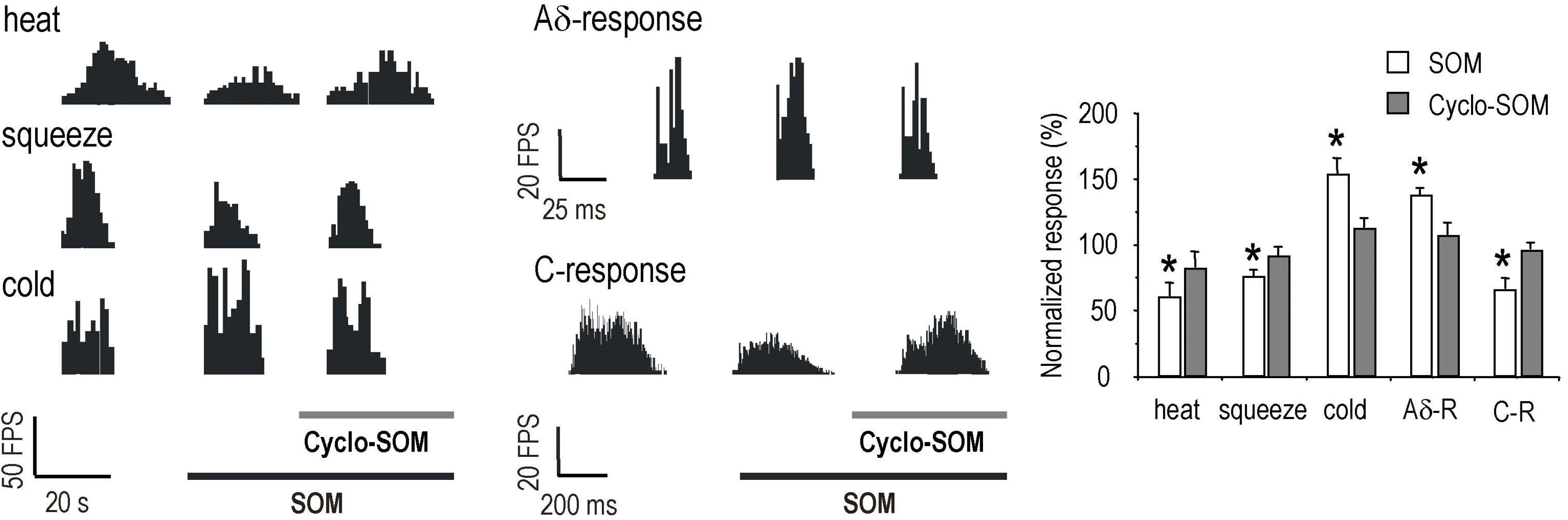 | Fig. 3.Blockade of SOM effect by SOM receptor antagonist, cyclo-SOM. The cyclo-SOM (100 nA) blocked the inhibitory effects of SOM (100 nA) on heat (59.2±9.8→80.8±11.5%, n=5) and squeeze (74.6±4.6→90.3±6.9%, n=6) as well as the facilitatory effect on cold stimulation (151.5±11.5→110.8±6.6%, n=4). In the case of activities by electrical stimuli such as Aδ – and C-response, SOM effect was also inhibited by cyclo-SOM (Aδ-response, 135.4±6.2→105.4±9.2%, n=5; C-response, 64.6±7.7→93.8±5.2%, n=5). Each bar graph represents mean value±standard error for SOM-and cyclo-SOM effect on noxious stimuli. The asterisk shows significant difference in SOM effect (paired t-test, p<0.05). |
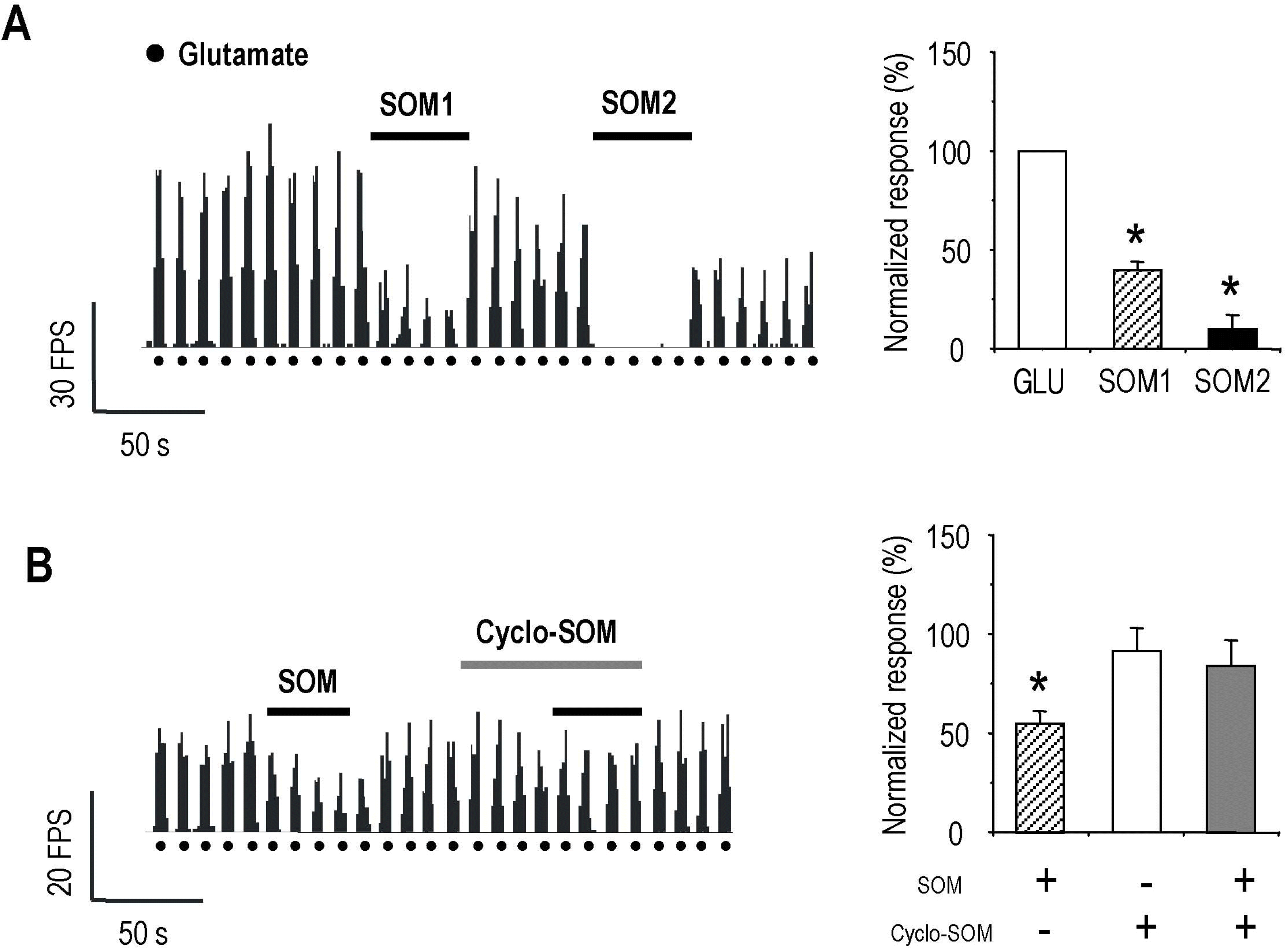 | Fig. 4.Effects of SOM on glutamate-evoked activity of RPSDH neuron. Iontophoretical application of GLU (100 nA every 5 seconds) induced the activity of RPSDH neuron. SOM has inhibitory action on the GLU-evoked activity of RPSDH neuron in a dose dependent manner (SOM1, 100 nA; SOM2, 200 nA). This inhibitory effect of SOM was blocked by cyclo-SOM (100 nA). Each bar graph represents mean value±standard error for SOM- and cyclo-SOM effect on glutamate-evoked activity of RPSDH neuron. Similarly, the asterisk shows significant difference in SOM effect (paired t-test, p<0.05). |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download