Abstract
KIF1Bβ is a member of the Kinesin superfamily proteins (KIFs), which are microtubule-dependent molecular motors that are involved in various intracellular organellar transport processes. KIF1Bβ is not restricted to neuronal systems, however, is widely expressed in other tissues, even though the function of KIF1Bβ is still unclear. To elucidate the KIF1Bβ-binding proteins in non-neuronal cells, we used the yeast two-hybrid system, and found a specific interaction of KIF1Bβ and the sorting nexin (SNX) 17. The C-terminal region of SNX17 is required for the binding with KIF1Bβ. SNX17 protein bound to the specific region of KIF1Bβ (813-916. aa), but not to other kinesin family members. In addition, this specific interaction was also observed in the Glutathione S-transferase pull-down assay. An antibody to SNX17 specifically co-immunoprecipitated KIF1Bβ associated with SNX17 from mouse brain extracts. These results suggest that SNX17 might be involved in the KIF1Bβ-mediated transport as a KIF1Bβ adaptor protein.
Go to : 
REFERENCES
Aizawa H., Sekine Y., Takemura R., Zhang Z., Nangaku M., Hirokawa N. Kinesin family in murine central nervous system. J Cell Biol. 119:1287–1296. 1992.

Bunn RC., Jensen MA., Reed BC. Protein interactions with the glucose transporter binding protein GLUT1CBP that provide a link between GLUT1 and the cytoskeleton. Mol Biol Cell. 10:819–832. 1999.

Burden JJ., Sun XM., Garcia AB., Soutar AK. Sorting motifs in the intracellular domain of the low density lipoprotein receptor interact with a novel domain of sorting nexin-17. J Biol Chem. 279:16237–16245. 2004.

Chishti AH., Kim AC., Marfatia SM., Lutchman M., Hanspal M., Jindal H., Liu SC., Low PS., Rouleau GA., Mohandas N., Chasis JA., Conboy JG., Gascard P., Takakuwa Y., Huang SC., Benz EJ Jr., Bretscher A., Fehon RG., Gusella JF., Ramesh V., Solomon F., Marchesi VT., Tsukita S., Tsukita S., Arpin M., Louvard D., Tonks NK., Anderson JM., Fanning AS., Bryant PJ., Woods DF., Hoover KB. The FERM domain: aunique module involved in the linkage of cytoplasmic proteins to the membrane. Trends Biochem Sci. 23:281–282. 1998.
Dorner C., Ullrich A., Haring HU., Lammers R. The kinesin-like motor protein KIF1C occurs in intact cells as a dimer and associates with proteins of the 14-3-3 family. J Biol Chem. 274:33654–33660. 1999.

Florian V., Schluter T., Bohnensack R. A new member of the sorting nexin family interacts with the C-terminus of P-selectin. Biochem Biophys Res Commun. 281:1045–1050. 2001.

Goldstein LS. Kinesin molecular motors: transport pathways, receptors, and human disease. Proc Natl Acad Sci U S A. 98:6999–7003. 2001.

Hirokawa N. Kinesin and dynein superfamily proteins and the mechanism of organelle transport. Science. 279:519–526. 1998.

Kanai Y., Okada Y., Tanaka Y., Harada A., Terada S., Hirokawa N. KIF5C, A novel neuronal kinesin enriched in motor neurons. J Neurosci. 20:6374–6384. 2000.

Karcher RL., Deacon SW., Gelfand VI. Motor-cargo interactions: the key to transport specificity. Trends Cell Biol. 12:21–27. 2002.

Kim SJ., Lee CH., Park HY., Yea SS., Jang WH., Lee SK., Park YH., Jung YW., Seog DH. Kinesin superfamily KIF1B alpha protein binds to the PDZ domain of MALS-3. The Korean Journal of Anatomy. 39:375–382. 2006.
Knauth P., Schluter T., Czubayko M., Kirsch C., Florian V., Schreckenberger S., Hahn H., Bohnensack R. Functions of sorting nexin 17 domains and recognition motif for P-selectin trafficking. J Mol Biol. 347:813–825. 2005.

Kondo S., Sato-Yoshitake R., Noda Y., Aizawa H., Nakata T., Matsuura Y., Hirokawa N. KIF3A is a new microtubule-based anterograde motor in the nerve axon. J Cell Biol. 125:1095–1107. 1994.

Miki H., Setou M., Kaneshiro K Hirokawa N. All kinesin superfamily protein, KIF, genes in mouse and human. Proc Natl Acad Sci USA. 98:7004–7011. 2001.

Mok H., Shin H., Kim S., Lee JR., Yoon J., Kim E. Association of the kinesin superfamily motor protein KIF1Balpha with postsynaptic density-95 (PSD-95), synapse-associated protein-97, and synaptic scaffolding molecule PSD-95/discs large/zona occludens-1 proteins. J Neurosci. 22:5253–5258. 2002.
Nakagawa T., Setou M., Seog D., Ogasawara K., Dohmae N., Takio K., Hirokawa N. A novel motor, KIF13A, transports mannose-6-phosphate receptor to plasma membrane through direct interaction with AP-1 complex. Cell. 103:569–581. 2000.

Nangaku M., Sato-Yoshitake R., Okada Y., Noda Y., Takemura R., Yamazaki H., Hirokawa N. KIF1B, a novel microtubule plus end-directed monomeric motor protein for transport of mitochondria. Cell. 79:1209–1220. 1994.

Okada Y., Higuchi H., Hirokawa N. Processivity of the single-headed kinesin KIF1A through biased binding to tubulin. Nature. 424:574–577. 2003.

Okada Y., Hirokawa N. A processive single-headed motor: kinesin superfamily protein KIF1A. Science. 283:1152–1157. 1999.

Okada Y., Yamazaki H., Sekine-Aizawa Y., Hirokawa N. The neuron-specific kinesin superfamily protein KIF1A is a unique monomeric motor for anterograde axonal transport of synaptic vesicle precursors. Cell. 81:769–780. 1995.
Paik JE., Kim N., Yea AA., Jang WH., Chung JY., Lee SK., Park YH., Han J., Seog DH. Kinesin superfamily KIF5 proteins bind to (III spectrin. Korean J Physiol Pharmacol. 8:167–172. 2004.
Sambrook J., Fritsch EF., Maniatis T. Molecular cloning: a laboratory manual. 3rd ed.Cold Spring Harbor Laboratory;Cold Spring Harbor, New York: 1989.
Seog DH., Lee DH., Lee SK. Molecular Motor Proteins of the Kinesin superfamily proteins (KIFs): structure, cargo and disease. J Korean Medical Science. 19:1–7. 2004.

Setou M., Nakagawa T., Seog DH., Hirokawa N. Kinesin superfamily motor protein KIF17 and mLin-10 in NMDA receptor-containing vesicle transport. Science. 288:1796–1802. 2000.

Setou M., Seog DH. Glutamate-receptor-interacting protein GRIP1 directly steers kinesin to dendrites. Nature. 417:83–87. 2002.

Su Q., Cai Q., Gerwin C., Smith CL., Sheng ZH. Syntabulin is a microtubule-associated protein implicated in syntaxin transport in neurons. Nat Cell Biol. 6:941–953. 2004.

Takeda S., Yamazaki H., Seog DH., Kanai Y., Terada S., Hirokawa N. Kinesin superfamily protein 3 (KIF3) motor transports fodrin-associating vesicles important for neurite building. J Cell Biol. 148:1255–1265. 2000.

van Kerkhof P., Lee J., McCormick L., Tetrault E., Lu W., Schoenfish M., Oorschot V., Strous GJ., Klumperman J., Bu G. Sorting nexin 17 facilitates LRP recycling in the early endosome. EMBO J. 24:2851–2861. 2005.

Wang Y., Zhou Y., Szabo K., Haft CR., Trejo J. Down-regulation of protease-activated receptor-1 is regulated by sorting nexin 1. Mol Biol Cell. 13:1965–1976. 2002.

Williams R., Schluter T., Roberts MS., Knauth P., Bohnensack R., Cutler DF. Sorting nexin 17 accelerates internalization yet retards degradation of P-selectin. Mol Biol Cell. 15:3095–3105. 2004.

Worby CA., Dixon JE. Sorting out the cellular functions of sorting nexins. Nat Rev Mol Cell Biol. 3:919–931. 2002.

Yonekawa Y., Harada A., Okada Y., Funakoshi T., Kanai Y., Takei Y., Terada S., Noda T., Hirokawa N. Defect in synaptic vesicle precursor transport and neuronal cell death in KIF1A motor protein-deficient mice. J Cell Biol. 141:431–441. 1998.

Zhao C., Takita J., Tanaka Y., Setou M., Nakagawa T., Takeda S., Yang HW., Terada S., Nakata T., Takei Y., Saito M., Tsuji S., Hayashi Y., Hirokawa N. Charcot-Marie-Tooth disease type 2A caused by mutation in a microtubule motor KIF1Bbeta. Cell. 105:587–597. 2001.
Go to : 
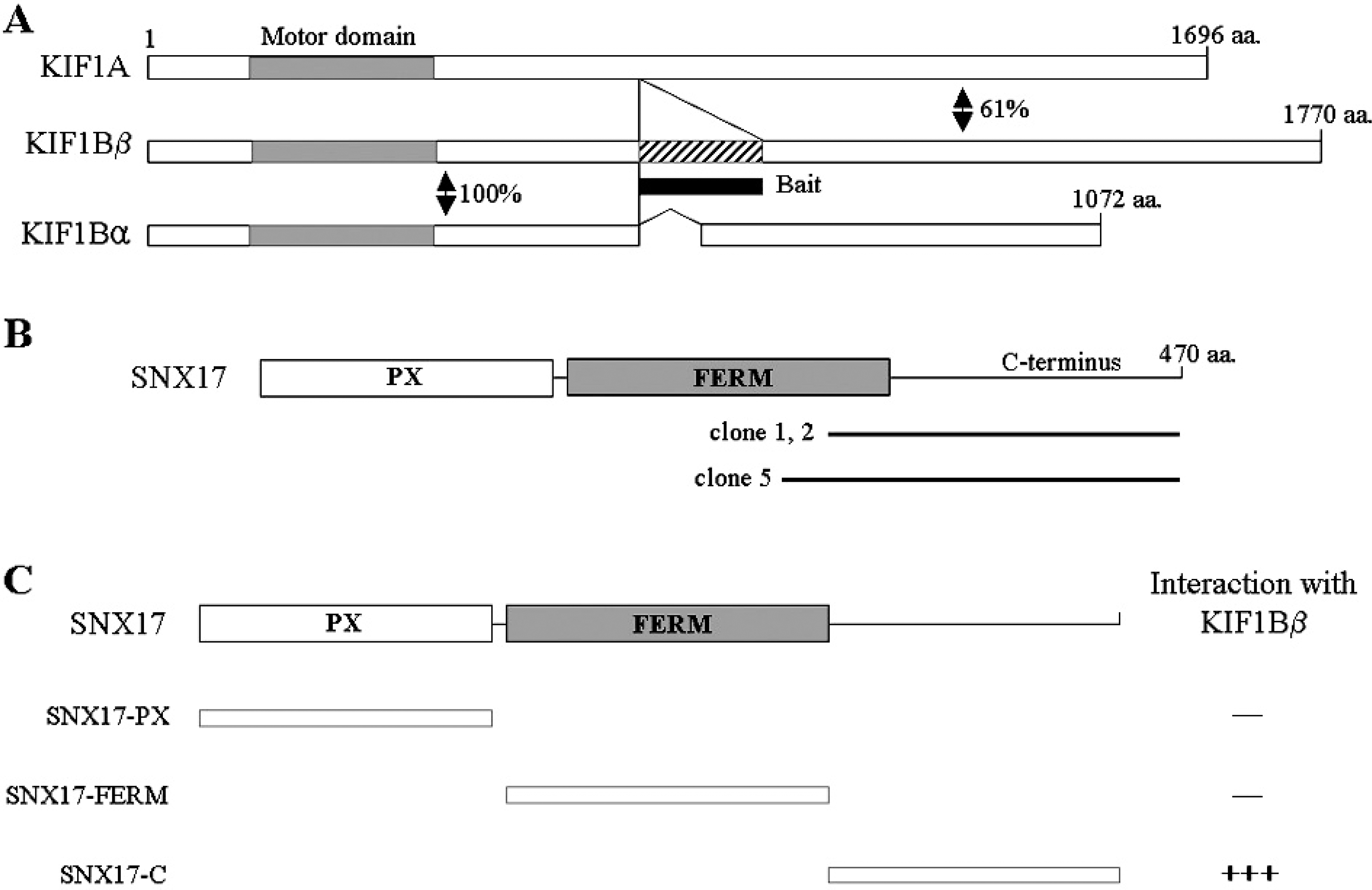 | Fig. 1.Identification of the proteins interacting with KIF1Bβ by yeast two-hybrid screening. (A) Schematic illustrations of KIF1A and KIF1B isoforms. Motor domain and KIF1Bβ specific region are indicated gray and hatched box, respectively. Amino acid homology in KIF1A and KIF1B isoforms are represented as a percentage. The positions of the amino acids are indicated. (B) Schematic diagram of domain structure of SNX17. The open box corresponds to PX domain and the filled box to the B41 (FERM) domain. Clone 1, 2 and 5 were found in yeast two-hybrid screen. Clone 1, 2 and 5 were overlapped at the C-terminal region of SNX17. aa, the amino acid residue number. (C) Schematic representation of the SNX17 truncation clones. Several truncated forms of SNX17 were tested in the yeast two-hybrid assay for interaction with KIF1B β. +, interaction with KIF1B β; -, no interaction with KIF1B β. |
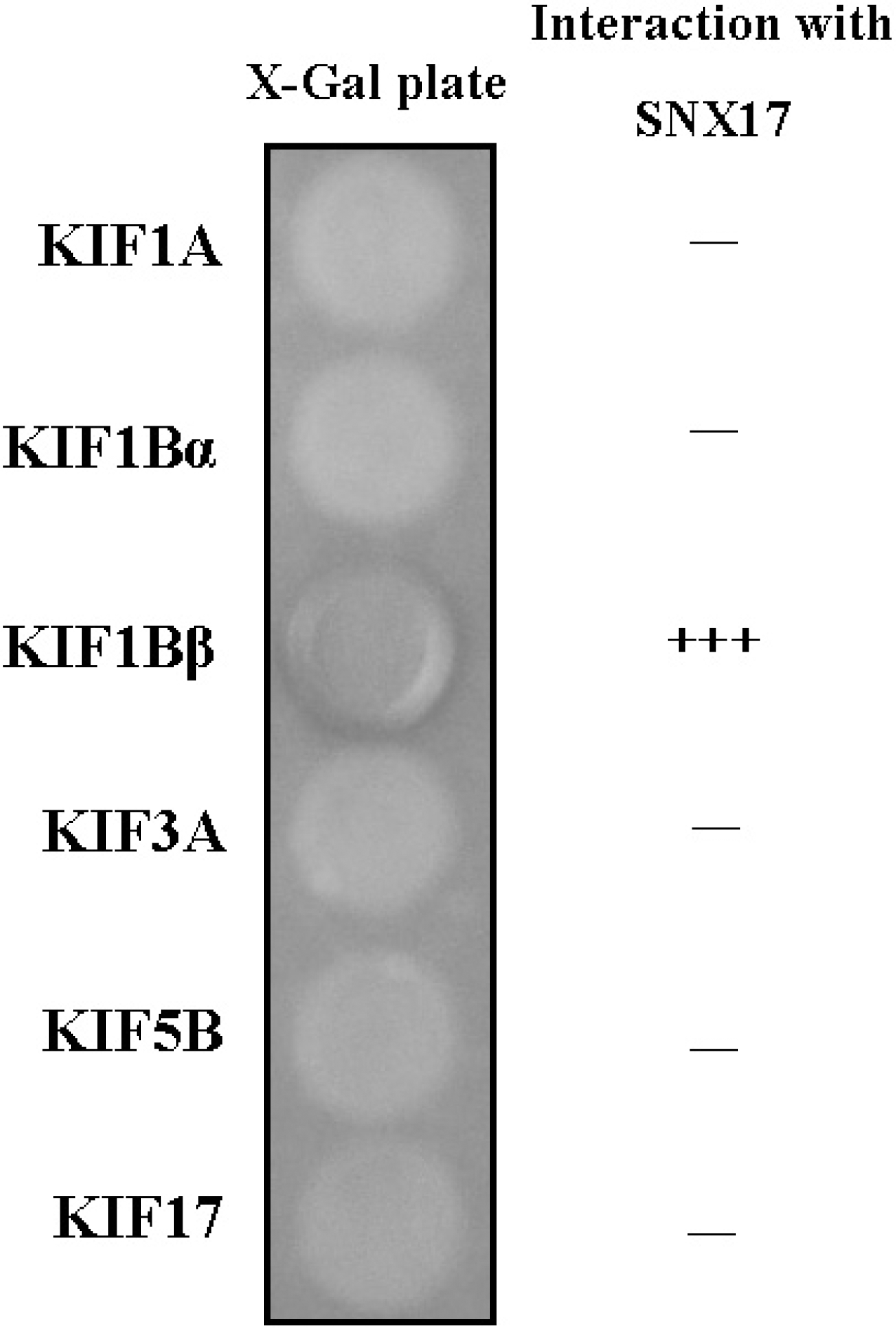 | Fig. 2.Interaction between KIFs and SNX17. The C-terminal regions of each KIF protein were fused to the pLexA DNA binding domain. SNX17 specifically interacted with KIF1B β, but not with KIF1A, KIF1B α, KIF3A, KIF5B, or KIF17 (+ + +, interaction with SNX17; -, no interaction with SNX17). |
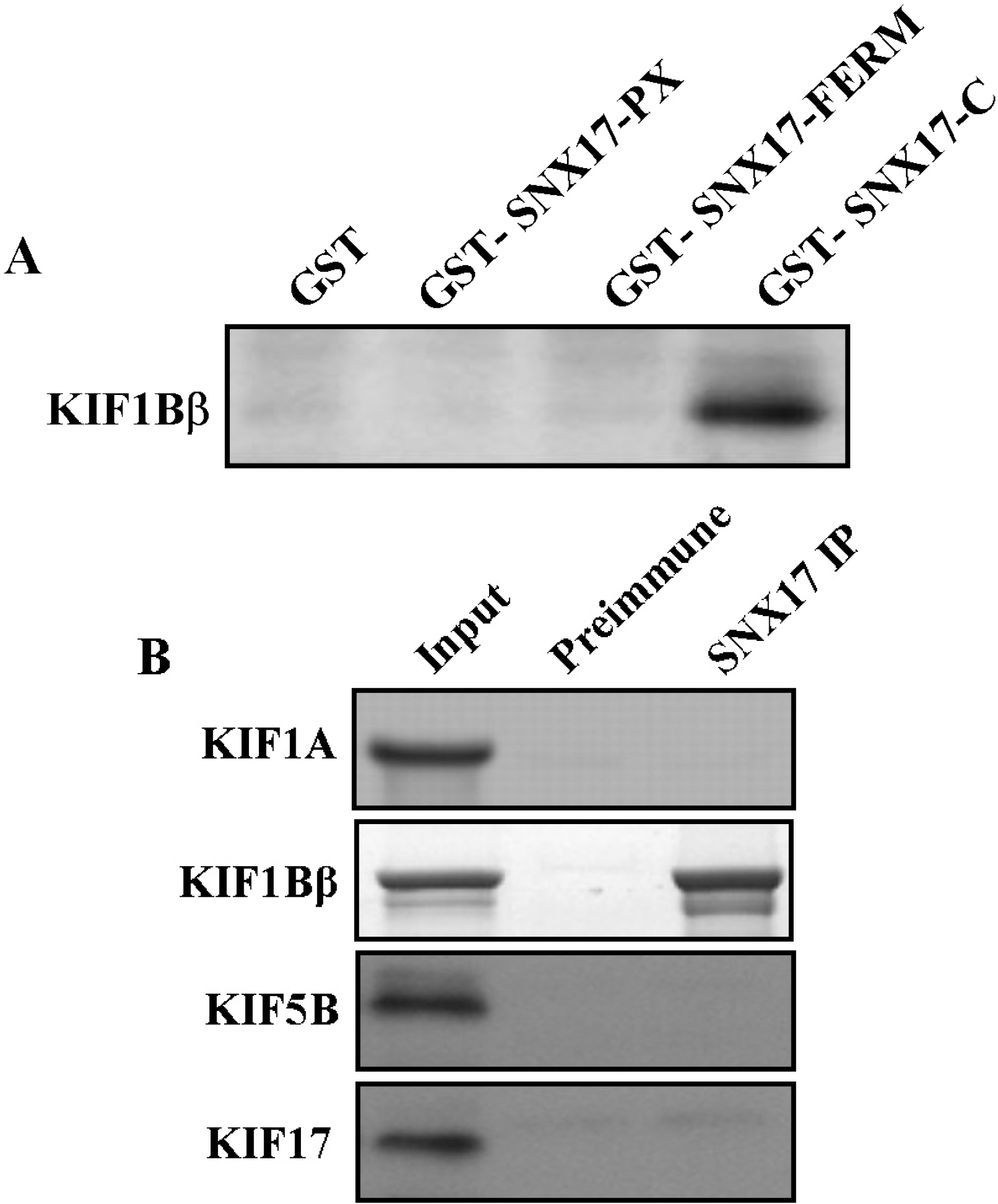 | Fig. 3.Association of KIF1B β with SNX17 in the GST pull-down assay and co-immunoprecipitation. (A) Proteins in the mouse brain lysate were allowed to bind to GST alone, GST-SNX17-PX, GST-SNX17-FERM and GST-SNX17-C fusion proteins. The elution fractions were resolved by SDS-PAGE, and Western blotting was performed using an antibody to KIF1Bβ. (B) Mouse brain lysates were immunoprecipitated with an anti-SNX17 antibody or preimmune serum, and the precipitates were immunoblotted with anti-KIF antibodies. Input: 10% of the mouse brain lysates used for each co-immunoprecipitation assay. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download