Abstract
The influence of alpha-fetoprotein (AFP) on the bone marrow (BM) natural suppressor (NS) cells of intact Ehrlich carcinoma -bearing CBA mice was studied. Bone marrow NS cells were fractionated into three fractions by isopycnic centrifugation on percoll gradients: NS1 (ρ = 1.080 g/ml), NS2 (ρ = 1.090 g/ml) and NS3 (1.100>ρ>1.090 g/ml). These fractions were highly different in their sensitivity to known NS cell inductors (interleukin (IL)-2, IL-3 or histamine). None of the NS fractions isolated from the intact mice spontaneously produced antiproliferative activity, however, they showed a high level of NS (antiproliferative and natural killer cell inhibitory) activity under the influence of AFP. A single injection of AFP to intact mice led to an increase of spontaneous NS activity and the inhibition of natural killer cell activity. NS activity, especially NS2, was increased in when tumor cells were subcutaneously inoculated three days after AFP injection. In the AFP-treated mice, the tumor mass at 14 days was 60% larger than that in the untreated mice. Our data confirmed that AFP is a tumor marker that can inhibit cancer immunity and plays a role in cancer pathogenesis.
Go to : 
REFERENCES
Abelev GI. Alpha-fetoprotein biology. Skulachev VP, editor. ed,. Soviet Science Review (section D) Physicochemical Biology. Harwood Acad Publ;New York: p. p. 85–109. 1993.
Angulo I., Rodriguez R., Garcia B., Medina M., Navarro J., Subiza JL. Involvement of nitric oxide in bone marrow-derived natural suppressor activity. J Immunol. 155:15–26. 1995.
Belyaev NN., Zakiryanova GK., Khegai LA., Beklemishev AB. Analysis of antimitogenic and histamine binding functions of bone marrow cell isopycnic fractions. Immunology. 3:15–17. 1993. (Rus.).
Cardoso E., Valdez G., Comini E., Matera L. Effect of human alpha-fetoprotein on native and in vitro-stimulated NK activity. J Clin Lab Immunol. 34:183–188. 1991.
Carmichael J., DeGraff WG., Gazdar AF., Minna JD., Mitchell JB. Evaluation of a tetrazolium-based semi-automated colorimetric assay: assessment of chemosensitivity testing. Cancer Research. 47:936–942. 1987.
Chakraborty M., Mandal C. Immuno-supressive effect of human alpha-fetoprotein: a cross species study. Immunol Invest. 22:329–339. 1993.
Holda JH., Maier T., Claman HN. Natural suppressor activity in graft-vs-host spleen and normal bone marrow is augmented by IL-2 and interferon-γ. J Immunol. 137:3538–3543. 1986.
Hoskin DW., Murgita RA. Specific maternal anti-fetal lymphoproliferative responses and their regulation by natural immunosuppressive factors. Clin Exp Immunol. 76:262–267. 1989.
Moore SC., Theus SA., Barnett JB., Soderberg LS. Cytokine regulation of bone marrow natural suppressor cell activity in the suppression of lymphocyte function. Cell Immunol. 141:398–408. 1992.

Semeniuk DJ., Boismenu R., Tam J., Weissenhofer W., Murgita RA. Evidence that immunosuppression is an intrinsic property of the alpha-fetoprotein molecule. Adv Exp Med Biol. 383:255–269. 1999.

Sinenko SA., Belyaev NN., Kuzovlev VD., Khakimzhanov AA. The immunochemical distinctions of human-α fetoprotein preparations as revealed with novel monoclonal antibodies. Reports MS-AS RK. 1:72–80. 1999.
Subiza JL., Vinuela JE., Rodriguez R., Gil J., Figueredo MA., De la Concha EG. Development of splenic natural suppressor (NS) cells in Ehrlich tumor-bearing mice. Int J Cancer. 44:307–314. 1989.

Sykes M., Strober S. Mechanisms of tolerance. Hematopoietic cell transplantation. 2nd ed.Blackwell Science;Malden: 1999.

Yamada A., Hayami M. Suppression of natural killer cell activity by chicken alpha-fetoprotein in Japanease quails. J Natl Cancer Inst. 70:735–738. 1983.
Yamaguchi V., Takashima I., Funakoshi M., Kawami H., Toge T. Defective natural killer activity in gastric cancer patients: possible involvement of suppressor factor receptor. In Vivo. 8:279–283. 1994.
Young MR., Young ME., Wright MA. Stimulation of immune-suppressive bone marrow cells by colony-stimulated factors. Exp Hematol. 18:806–811. 1990.
Young MR., Wright MA., Coogan M., Young ME., Bagash J. Tumor-derived cytokines induce bone marrow suppressor cells that mediate immunosuppression through transforming growth factor beta. Cancer Immunol Immunother. 35:14–18. 1992.
Go to : 
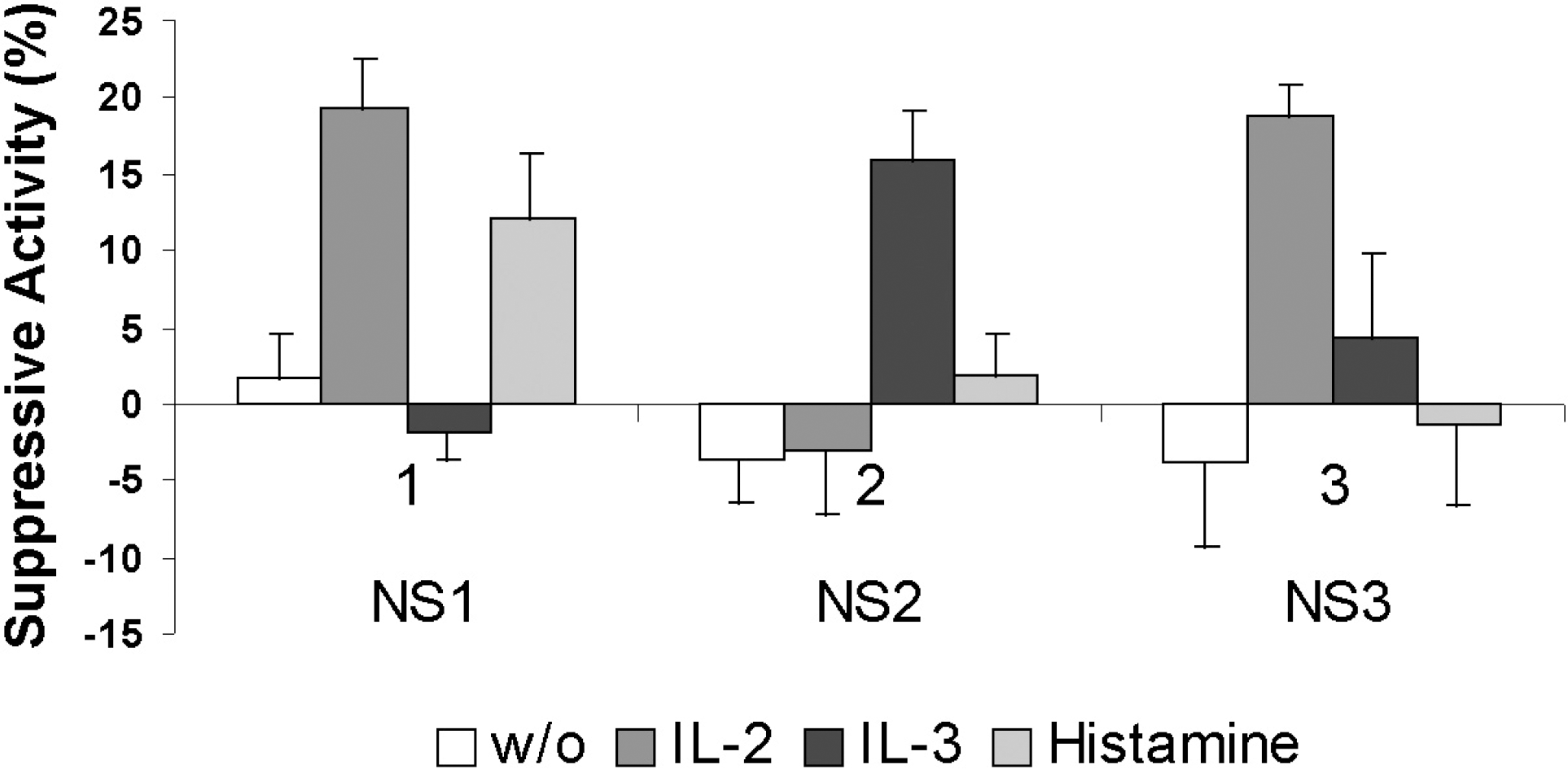 | Fig. 1.Production of suppression factors (suppressive activity) by three NS cell fractions with and without (w/o) influence of inducers. Data are shown as mean±SD. |
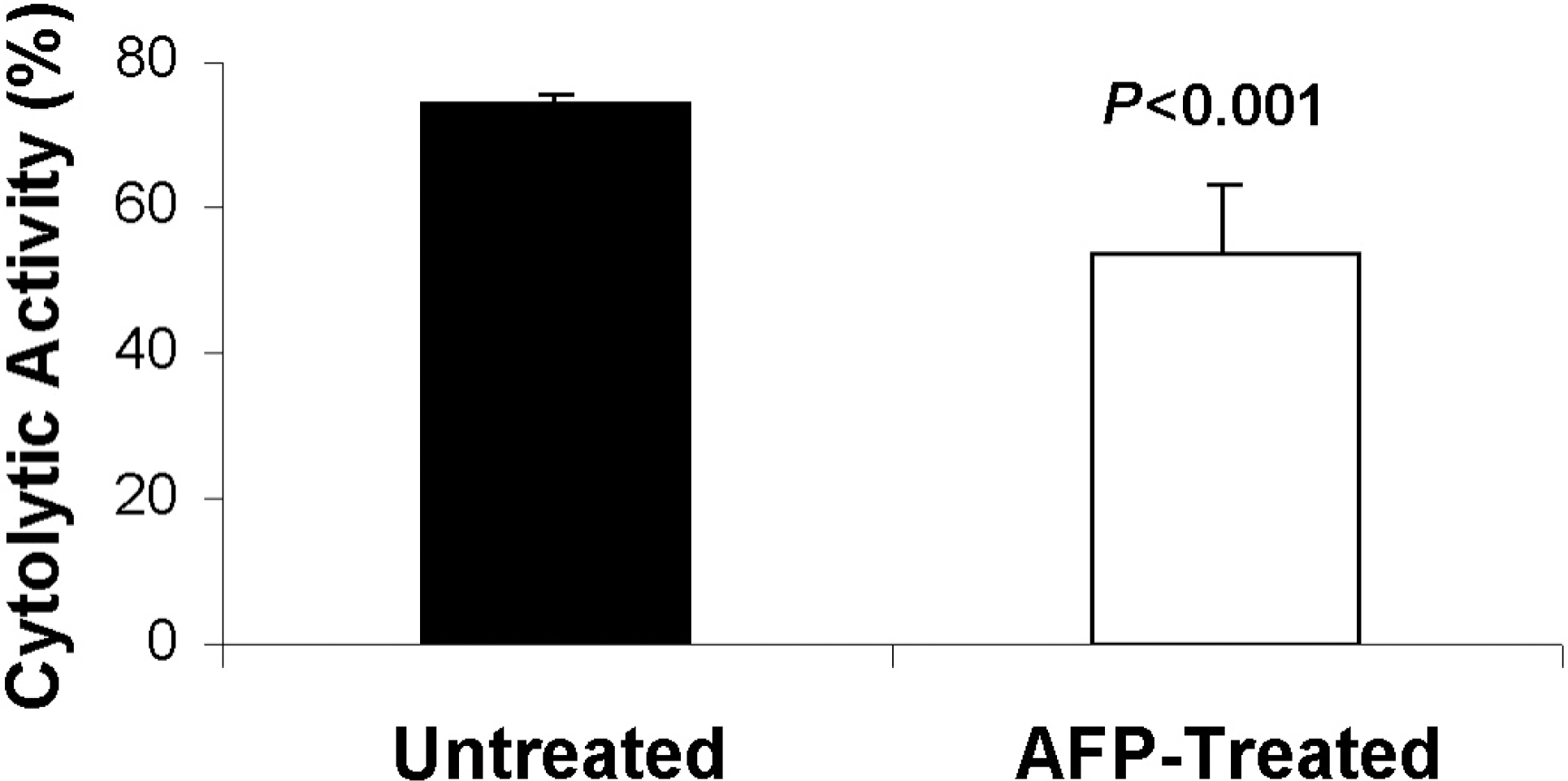 | Fig. 2.Cytolytic activity of spleen NK cells obtained from untreated and AFP-treated mice. Data are shown as mean±SD. |
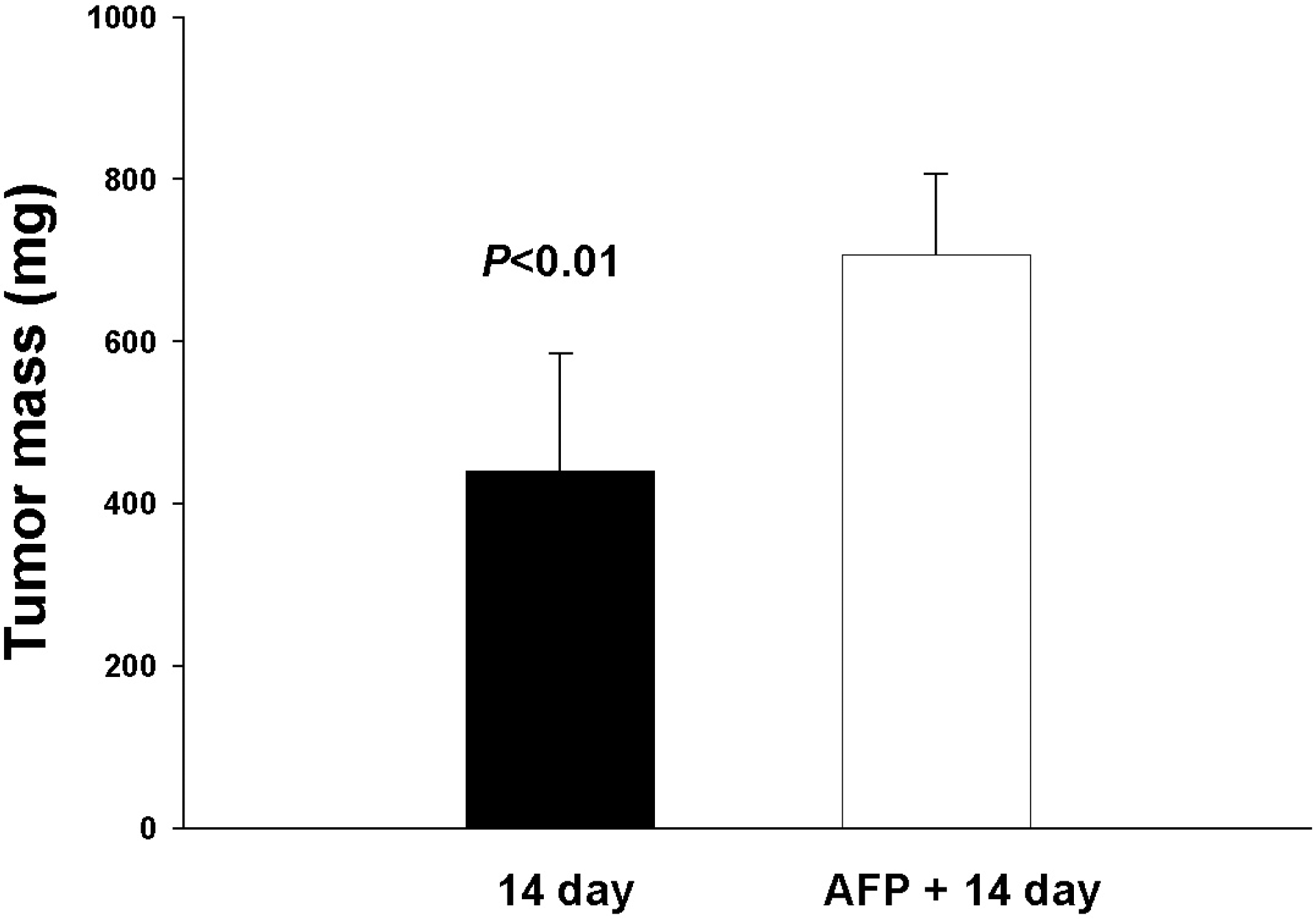 | Fig. 3.Influence of a single AFP injection on Ehrlich carcinoma (EC) growth. Tumor mass was observed with and without AFP injection after EC inoculation at the 14th day. Data are shown as mean±SD. |
Table 1.
Antiproliferative and NK inhibitory activity of NS cell fractions. Suppressive activity of different NS cell fractions either under influence or without (w/o) influence of AFP, which inhibits both myeloma cell proliferation (SA) and NK cytolytic activity (CLA)
| Cell targets for suppressive factors | SA/CLA, % | |||||
|---|---|---|---|---|---|---|
| NS1 | NS2 | NS3 | ||||
| w/o | AFP | w/o | AFP | w/o | AFP | |
| Myeloma | 3.8±7.7 | 50.6±1.2 | 2.4±8.1 | 52.6±1.4 | −2.5±4.7 | 43.5±2.4 |
| P (w/o) | 0.001 | 0.001 | 0.001 | |||
| P (NS3) | 0.005 | 0.002 | ||||
| NK∗ | 73.5±4.0 | 55.6±2.3 | 77.0±2.4 | 42.9±3.4 | 72.6±2.6 | 58.8±4.1 |
| P (w/o) | 0.005 | 0.001 | 0.012 | |||
| P (NS2) | 0.008 | 0.007 | ||||
Table 2.
Spontaneous or AFP-induced NS activity after EC cell inoculation or AFP injection. Suppressive activity (SA) to inhibit myeloma cell proliferation by different NS cell fractions obtained from EC bearing – or intact mice after a single AFP injection




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download