Abstract
In the present study, we aimed to identify the synergistic effects of concurrent treatment of low concentrations of cilostazol and probucol to inhibit the oxidative stress with suppression of inflammatory markers in the cultured human coronary artery endothelial cells (HCAECs). Combination of cilostazol (0.3 ~ 3 μ M) with probucol (0.03 ~ 0.3 μ M) significantly suppressed TNF-α-stimulated NAD(P)H-dependent superoxide, lipopolysaccharide (LPS)-induced intracellular reactive oxygen species (ROS) production and TNF-α release in comparison with probucol or cilostazol alone. The combination of cilostazol (0.3 ~ 3 μ M) with probucol (0.1 ~ 0.3 μ M) inhibited the expression of vascular cell adhesion molecule-1 (VCAM-1) and monocyte chemoattractant protein-1 (MCP-1) more significantly than did the monotherapy with either probucol or cilostazol. In line with these results, combination therapy significantly suppressed monocyte adhesion to endothelial cells. Taken together, it is suggested that the synergistic effectiveness of the combination therapy with cilostazol and probucol may provide a beneficial therapeutic window in preventing atherosclerosis and protecting from cerebral ischemic injury.
Go to : 
REFERENCES
Bevilacqua MP., Nelson RM., Mannori G., Cecconi O. Endothelialleukocyte adhesion molecules in human disease. Annu Rev Med. 45:361–378. 1994.
Bilenko MV., Khil'chenko AV., Konovalova GG., Lankin VZ. Effect of antioxidant probucol on cell-mediated LDL oxidation in vitro and in vivo. Bull Exp Biol Med. 136:126–128. 2003.
Carew TE., Schwenke DC., Steinberg D. Antiatherogenic effect of probucol unrelated to its hypocholesterolemic effect: evidence that antioxidants in vivo can selectively inhibit low density lipoprotein degradation in macrophage-rich fatty streaks and slow the progression of atherosclerosis in the Watanabe heritable hyperlipidemic rabbit. Proc Natl Acad Sci USA. 84:7725–7772. 1987.

Choi JM., Shin HK., Kim KY., Lee JH., Hong KW. Neuroprotective effect of cilostazol against focal cerebral ischemia via antiapoptotic action in rats. J Pharmacol Exp Ther. 300:787–793. 2002.

Cybulsky MI., Iiyama K., Li H., Zhu S., Chen M., Iiyama M., Davis V., Gutierrez-Ramos J-C., Connelly PW., Milstone DS. A major role for VCAM-1, but not ICAM-1, in early atherosclerosis. J Clin Invest. 107:1255–1262. 2001.

Dansky HM., Barlow CB., Lominska C., Sikes JL., Kao C., Weinsaft J., Cybulsky MI., Smith JD. Adhesion of monocytes to arterial endothelium and initiation of atherosclerosis are critically dependent on vascular cell adhesion molecule-1 gene dosage. Arterioscler Thromb Vasc Biol. 21:662–1667. 2001.

Faruqi R., de la Motte C., DiCorleto PE. Alpha-tocopherol inhibits agonist-induced monocytic cell adhesion to cultured human endothelial cells. J Clin Invest. 94:92–600. 1994.

Ferns GA., Forster L., Stewart-Lee A., Nourooz-Zadeh J., Anggard EE. Probucol inhibits mononuclear cell adhesion to vascular endothelium in the cholesterol-fed rabbit. Atherosclerosis. 100:171–181. 1993.

Fruebis J., Gonzalez V., Silvestre M., Palinski W. Effect of probucol treatment on gene expression of VCAM-1, MCP-1, and M-CSF in the aortic wall of LDL receptor-deficient rabbits during early atherogenesis. Arterioscler Thromb Vasc Biol. 17:1289–1302. 1997.

Fruebis J., Silvestre M., Shelton D., Napoli C., Palinski W. Inhibition of VCAM-1 expression in the arterial wall is shared by structurally different antioxidants that reduce early atherosclerosis in NZW rabbits. J Lipid Res. 40:1958–1966. 1999.

Gu L., Okada Y., Clinton SK., Gerard C., Sukhova GK., Libby P., Rollins BJ. Absence of monocyte chemoattractant protein-1 reduces atherosclerosis in low density lipoprotein receptor-deficient mice. Mol Cell. 2:275–281. 1998.

Hong KW., Kim KY., Shin HK., Lee JH., Choi JM., Kwak YG., Kim CD., Lee WS., Rhim BY. Cilostazol prevents tumor necrosis factor-alpha-induced cell death by suppression of phosphatase and tensin homolog deleted from chromosome 10 phosphorylation and activation of Akt/cyclic AMP response element-binding protein phosphorylation. J Pharmacol Exp Ther. 306:1182–1190. 2003.
Johansson J., Olsson AG., Bergstrand L., Elinder LS., Nilsson S., Erikson U., Molgoard J., Holme I., Walldius G. Lowering of HDL2b by probucol partly explains the failure of the drug to affect femoral atherosclerosis in subjects with hypercholesterolemia. A Probucol Quantitative Regression Swedish Trial (PQRST) Report. Arterioscler Thromb Vasc Biol. 15:1049–1056. 1995.
Kim KY., Shin HK., Choi JM., Hong KW. Inhibition of lipopoly-saccharide-induced apoptosis by cilostazol in human umbilical vein endothelial cells. J Pharmacol Exp Ther. 300:709–715. 2002.

Kimura Y., Tani T., Kanbe T., Watanabe K. Effect of cilostazol on platelet aggregation and experimental thrombosis. Arzneimittelforschung. 35:1144–1149. 1985.
Kockx MM. Apoptosis in the atherosclerotic plaque: quantitative and qualitative aspects. Arterioscler Thromb Vasc Biol. 18:1519–1522. 1998.
Kuzuya M., Kuzuya F. Probucol as an antioxidant and antiatherogenic drug. Free Radic Biol Med. 14:67–77. 1993.

Li PF., Dietz R., von Harsdorf R. Differential effect of hydrogen peroxide and superoxide anion on apoptosis and proliferation of vascular smooth muscle cells. Circulation. 96:3602–3609. 1997.

Mallikaarjun S., Forbes WP., Bramer SL. Interaction potential and tolerability of the coadministration of cilostazol and aspirin. Clin Pharmacokinet. 37(Suppl 2):87–93. 1999.

Park SY., Lee JH., Kim CD., Lee WS., Park WS., Han J., Kwak Y-G., Kim KY., Hong KW. Cilostazol suppresses superoxide production and expression of adhesion molecules in human endothelial cells via mediation of cAMP-dependent protein kinase-mediated maxi-K channel activation. J Pharmacol Exp Ther. 317:1238–1245. 2006.

Park SY., Lee JH., Kim YK., Kim CD., Rhim BY., Lee WS., Hong KW. Beneficial synergistic effects of concurrent treatment with cilostazol and probucol against focal cerebral ischemic injury in rats. Brain Res. 1157:112–120. 2007.

Park SY., Lee JH., Kim YK., Kim CD., Rhim BY., Lee WS., Hong KW. Cilostazol prevents remnant lipoprotein particle-induced monocyte adhesion to endothelial cells by suppression of adhesion molecules and monocyte chemoattractant protein-1 expression via lectin-like receptor for oxidized low-density lipoprotein receptor activation. J Pharmacol Exp Ther. 312:1241–1248. 2005.

Reinoehl J., Frankovich D., Machado C., Kawasaki R., Baga JJ., Pires LA., Steinman RT., Fromm BS., Lehmann MH. Probucol-associated tachyarrhythmic events and QT prolongation: importance of gender. Am Heart J. 131:1184–1191. 1996.

Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 362:801–809. 1993.

Sekiya M., Funada J., Watanabe K., Miyagawa M., Akutsu H. Effects of probucol and cilostazol alone and in combination on frequency of poststenting restenosis. Am J Cardiol. 82:144–147. 1998.

Shin HK., Kim YK., Kim KY., Lee JH., Hong KW. Remnant lipoprotein particles induce apoptosis in endothelial cells by NAD(P)H oxidase-mediated production of superoxide and cytokines via lectin-like oxidized low-density lipoprotein receptor-1 activation: prevention by cilostazol. Circulation. 109:1022–1028. 2004.
Tardif JC., Cote G., Lesperance J., Bourassa M., Lambert J., Doucet S., Bilodeau L., Nattel S., de Guise P. Probucol and multivitamins in the prevention of restenosis after coronary angioplasty. Multivitamins and Probucol Study Group. N Engl J Med. 337:365–372. 1997.
Go to : 
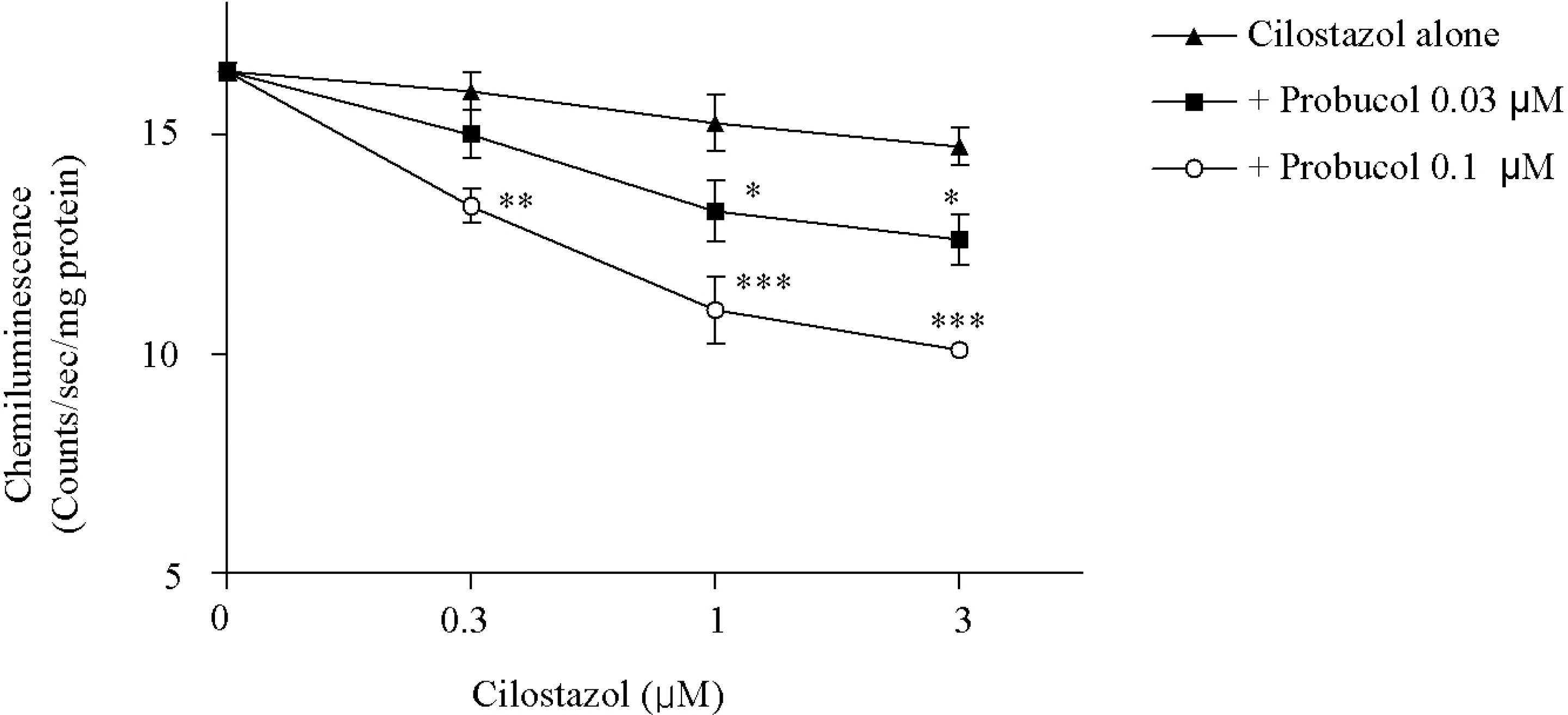 | Fig. 1.Effects of cilostazol alone and in combination of cilostazol with probucol on the NAD(P)H-dependent superoxide production in the HCAECs stimulated by TNF-α (50 ng/ml). The significant effect was evident by cilostazol in combination with probucol. Results are expressed as mean±S.E.M. of four experiments. ∗p<0.05, ∗∗p< 0.01, ∗∗∗p<0.001 vs. cilostazol alone. Significant differences were shown between cilostazol alone and cilostazol plus probucol 0.03 μM or cilostazol plus 0.1 μM probucol groups by two-way repeated measures ANOVA. |
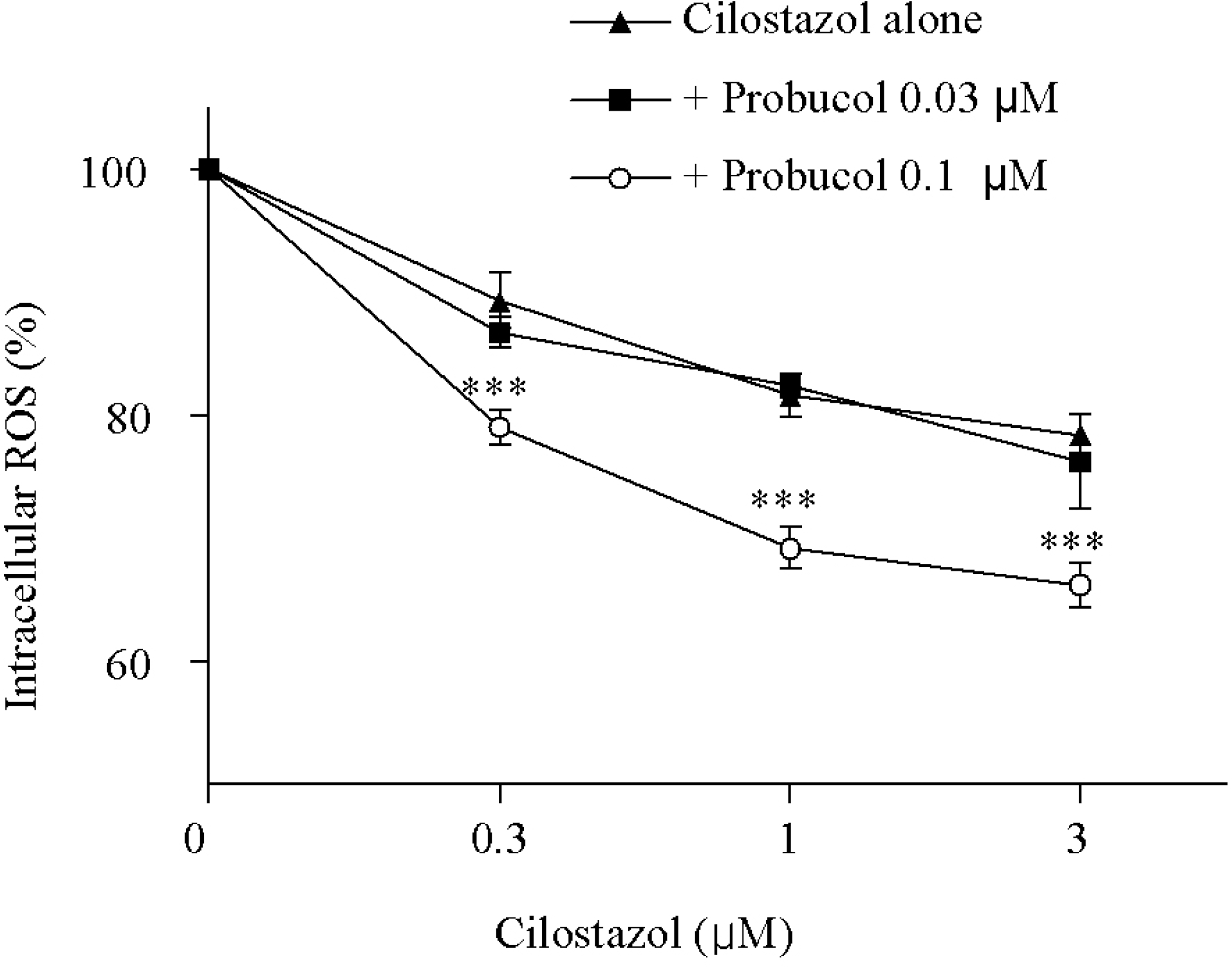 | Fig. 2.Inhibitory effect of cilostazol in combination with probucol on the intracellular ROS levels stimulated by in the HCAECs LPS (1μg/ml). LPS-stimulated intracellular ROS was significantly decreased by cilostazol plus 0.1 μM probucol in combination. ∗∗∗p <0.001 vs. cilostazol alone. Results are expressed as mean±S.E.M. of four experiments. Significant differences were shown between cilostazol alone and cilostazol plus 0.1μM probucol groups by two-way repeated measures ANOVA. |
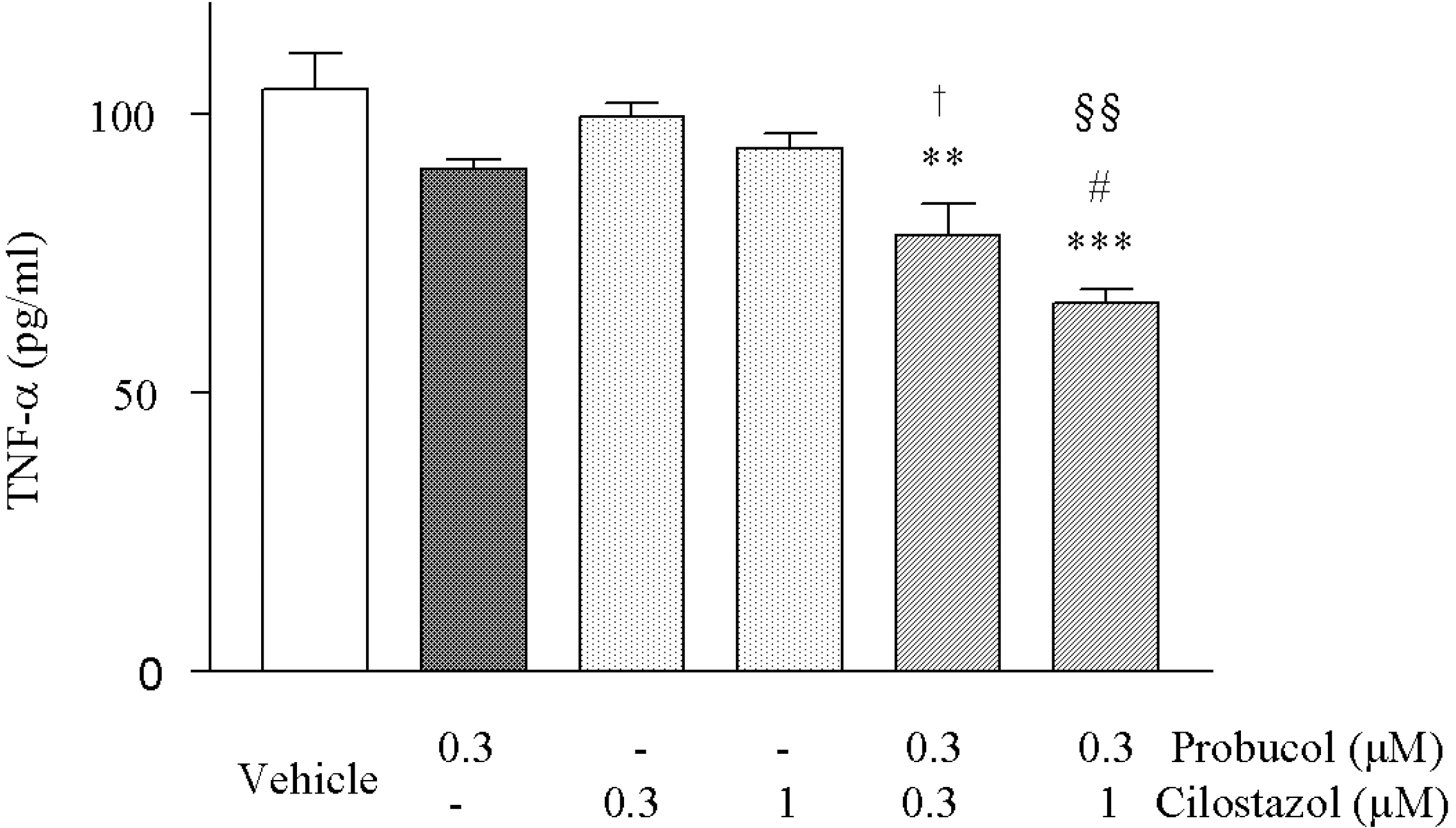 | Fig. 3.Effects of cilostazol and probucol alone and their combination on the TNF-α formation stimulated by in the HCAECs LPS (1 μ g/ml). Increased TNF-α by LPS was significantly decreased by cilostazol and probucol in combination. Results are expressed as mean±S.E.M. of three experiments. ∗∗p<0.01, ∗∗∗p<0.001 vs. vehicle, #p<0.05 vs. 0.3 μM probucol alone, †p<0.05 vs. 0.3 μM cilostazol alone, §§p<0.01 vs. 1 μM cilostazol alone. |
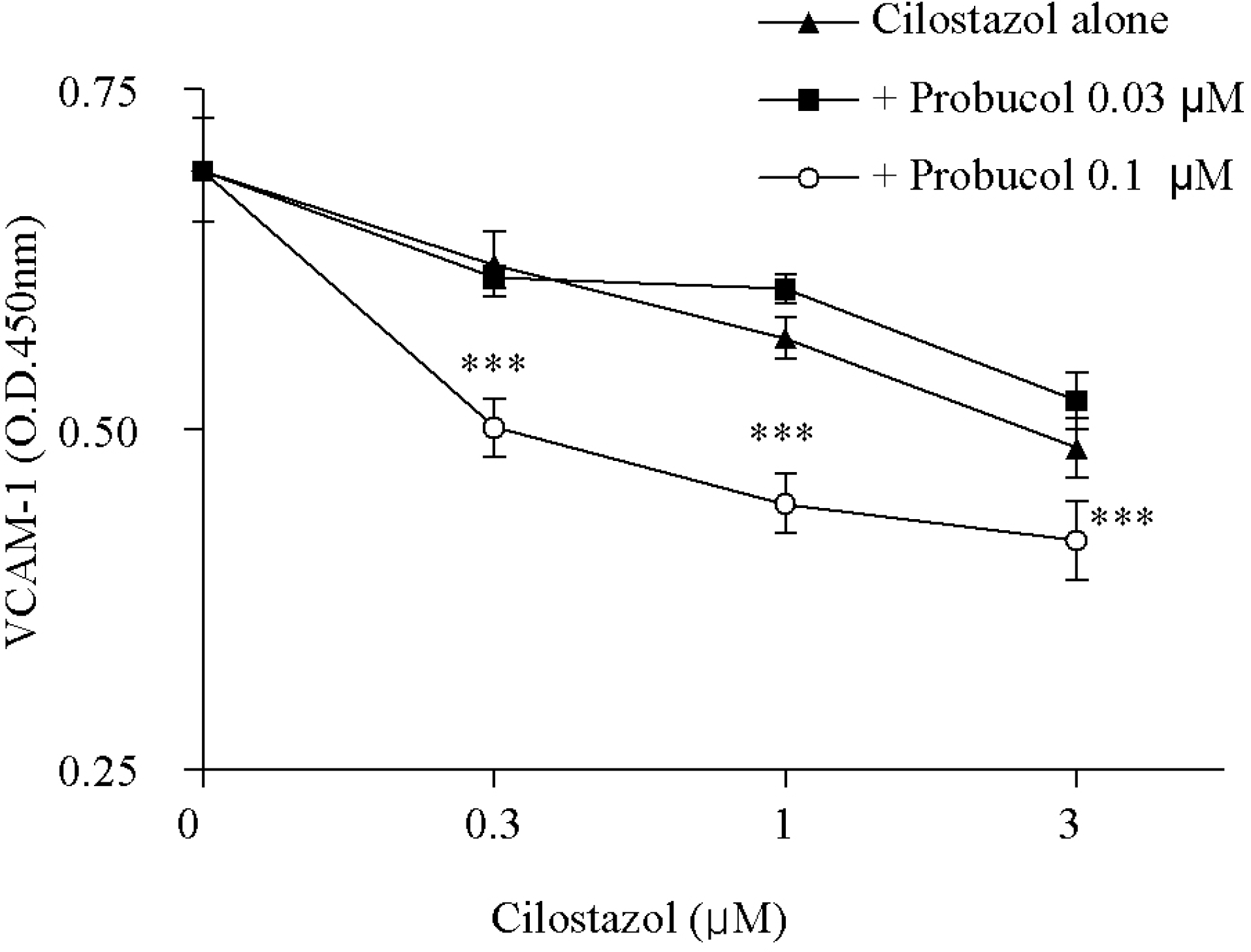 | Fig. 4.Inhibitory effect of cilostazol in combination with probucol on the VCAM-1expression in the HCAECs. TNF-α (50 ng/ml)-induced increased VCAM-1expression was significantly decreased by treatment with cilostazol and 0.1 μ M probucol in combination. Results are expressed as mean±S.E.M. of four experiments. ∗∗∗p<0.001 vs. cilostazol alone. Significant differences were shown between cilostazol plus 0.03 μM probucol and cilostazol plus 0.1 μ M probucol groups by two-way repeated measures ANOVA. |
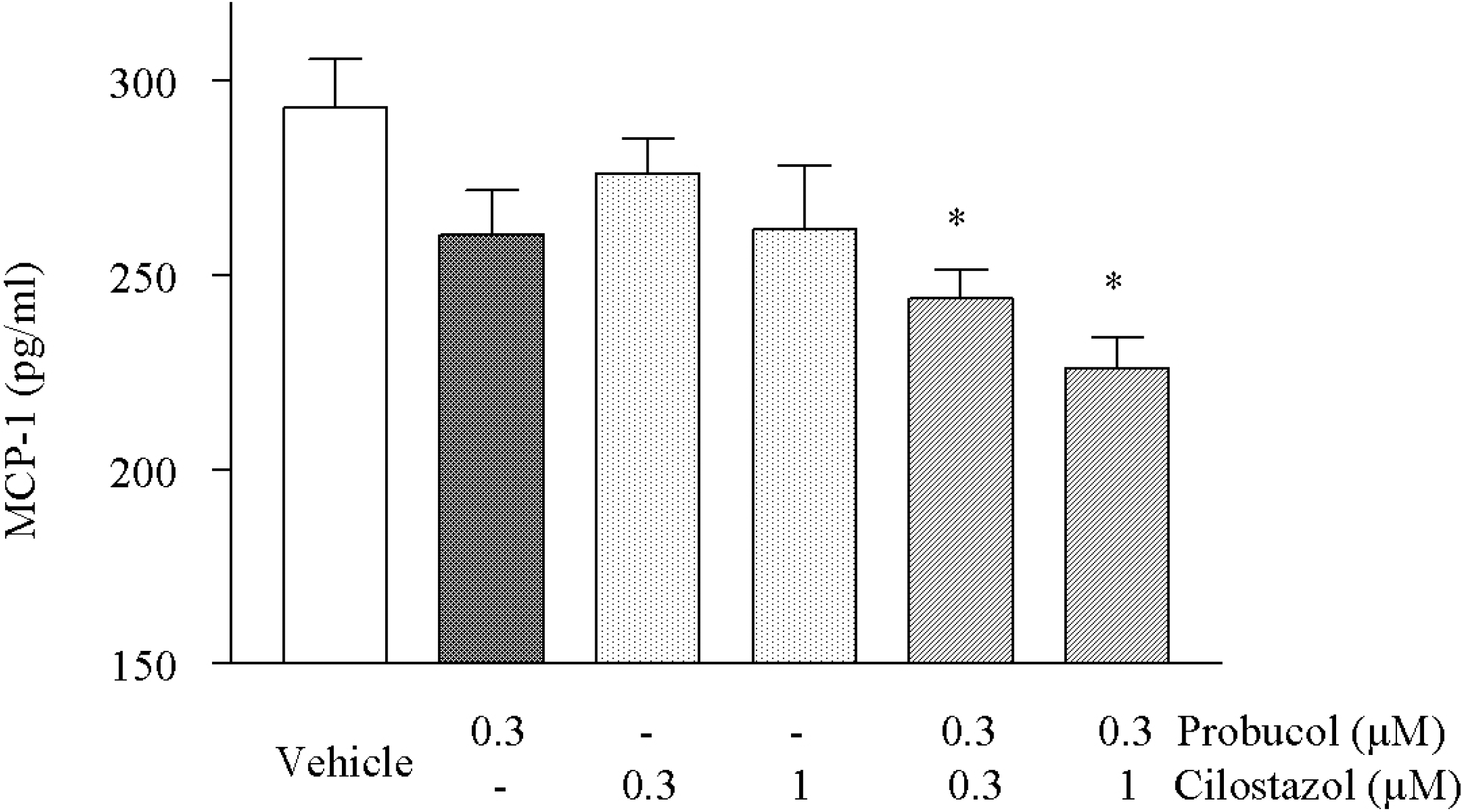 | Fig. 5.Effects of cilostazol and probucol in combination on the MCP-1 expression stimulated in the HCAECs by TNF-α (50 ng/ml). Concurrent treatment with 0.3 μM probucol plus 0.3 or 1 μM cilostazol significantly decreased MCP-1 expression in comparison to effect of probucol and cilostazol alone. Results are expressed as mean±S.E.M. of three experiments. ∗p<0.05 vs. vehicle. |
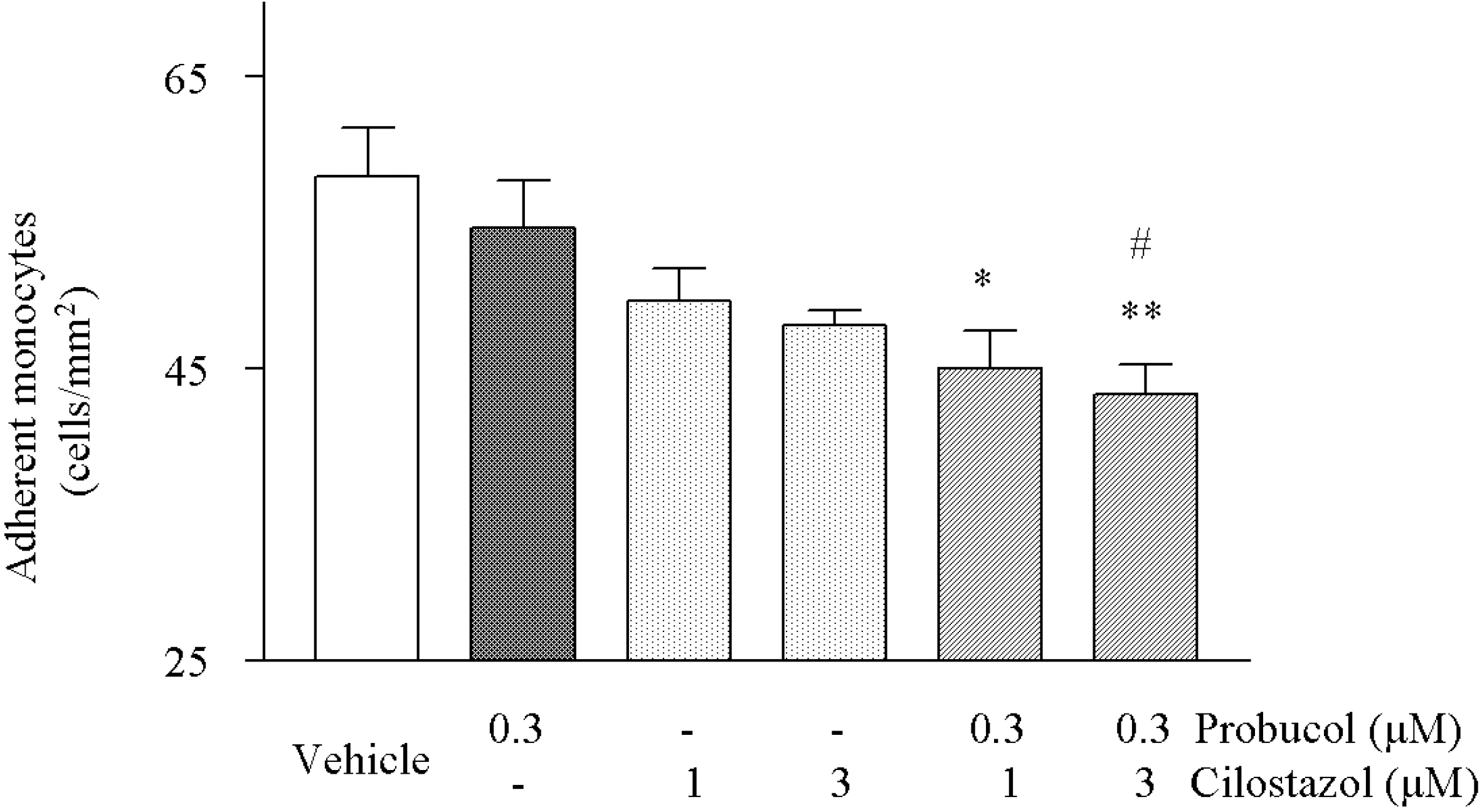 | Fig. 6.Effects of cilostazol and probucol alone and their combination on the monocyte adhesion to HCAECs. TNF-α (50 ng/ml)-stimulated adherent monocytes were significantly decreased by treatment with cilostazol and probucol in combination. Results are expressed as mean±S.E.M. of four experiments. ∗p<0.05, ∗∗p<0.01 vs. vehicle, #p<0.05 vs. probucol alone. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download