Abstract
Although growth associated protein-43 (GAP-43) is known to play a significant role in the regulation of axonal growth and the formation of new neuronal connections in the hippocampus, there is only a few studies on the effects of acute stress on GAP-43 mRNA expression in the hippocampus. Moreover, the effects of repeated citalopram treatment on chronic mild stress (CMS)-induced changes in GAP-43 mRNA expression in the hippocampus have not been explored before. To explore this question, male rats were exposed to acute immobilization stress or CMS. Also, citalopram was given prior to stress everyday during CMS procedures. Acute immobilization stress significantly increased GAP-43 mRNA expression in all subfields of the hippocampus, while CMS significantly decreased GAP-43 mRNA expression in the dentate granule cell layer (GCL). Repeated citalopram treatment decreased GAP-43 mRNA expression in the GCL compared with unstressed controls, but this decrease was not further potentiated by CMS exposure. Similar decreases in GAP-43 mRNA expression were observed in CA1, CA3 and CA4 areas of the hippocampus only after repeated citalopram treatment in CMS-exposed rats. This result indicates that GAP-43 mRNA expression in the hippocampus may differently respond to acute and chronic stress, and that repeated citalopram treatment does not change CMS-induced decreases in GAP-43 mRNA expression in the GCL.
Go to : 
REFERENCES
Bartanusz V., Aubry JM., Pagliusi S., Jezova D., Baffi J., Kiss JZ. Stress-induced changes in messenger RNA levels of N-methyl-D-aspartate and AMPA receptor subunits in selected regions of the rat hippocampus and hypothalamus. Neuroscience. 66:247–252. 1995.

Bekris S., Antoniou K., Daskas S., Papadopoulou-Daifoti Z. Behavioural and neurochemical effects induced by chronic mild stress applied to two different rat strains. Behav Brain Res. 161:45–59. 2005.

Bendotti C., Baldessari S., Pende M., Southgate T., Guglielmetti F., Samanin R. Relationship between GAP-43 expression in the dentate gyrus and synaptic reorganization of hippocampal mossy fibres in rats treated with kainic acid. Eur J Neurosci. 9:93–101. 1997.

Benowitz LI., Routtenberg A. GAP-43: an intrinsic determinant of neuronal development and plasticity. Trends Neurosci. 20:84–91. 1997.

Boyer PA., Skolnick P., Fossom LH. Chronic administration of imipramine and citalopram alters the expression of NMDA receptor subunit mRNAs in mouse brain. A quantitative in situ hybridization study. J Mol Neurosci. 10:219–233. 1998.
Cantallops I., Routtenberg A. Rapid induction by kainic acid of both axonal growth and F1/GAP-43 protein in the adult rat hippocampal granule cells. J Comp Neurol. 366:303–319. 1996.

Chen B., Wang JF., Sun X., Young LT. Regulation of GAP-43 expression by chronic desipramine treatment in rat cultured hippocampal cells. Biol Psychiatry. 53:530–537. 2003.

De Kloet ER., Kovacs GL., Szabo G., Telegdy G., Bohus B., Versteeg DH. Decreased serotonin turnover in the dorsal hippocampus of rat brain shortly after adrenalectomy: selective normalization after corticosterone substitution. Brain Res. 239:659–663. 1982.

Duman RS. Pathophysiology of depression: the concept of synaptic plasticity. Eur Psychiatry. 17(Suppl 3):306–310. 2002.

Ferrini M., Bisagno V., Piroli G., Grillo C., Deniselle MC., De Nicola AF. Effects of estrogens on choline-acetyltransferase immunoreactivity and GAP-43 mRNA in the forebrain of young and aging male rats. Cell Mol Neurobiol. 22:289–301. 2002.
Gold SJ., Ni YG., Dohlman HG., Nestler EJ. Regulators of G-protein signaling (RGS) proteins: region-specific expression of nine subtypes in rat brain. J Neurosci. 17:8024–8037. 1997.

Goodnick PJ., Goldstein BJ. Selective serotonin reuptake inhibitors in affective disorders–I. Basic pharmacology. J Psychopharmacol. 12:S5–20. 1998.
Gronli J., Fiske E., Murison R., Bjorvatn B., Sorensen E., Ursin R., Portas CM. Extracellular levels of serotonin and GABA in the hippocampus after chronic mild stress in rats. A microdialysis study in an animal model of depression. Behav Brain Res. 181:42–51. 2007.
Harvey BH., Jonker LP., Brand L., Heenop M., Stein DJ. NMDA receptor involvement in imipramine withdrawal-associated effects on swim stress, GABA levels and NMDA receptor binding in rat hippocampus. Life Sci. 71:43–54. 2002.

Herman JP., Cullinan WE. Neurocircuitry of stress: central control of the hypothalamo-pituitary-adrenocortical axis. Trends Neurosci. 20:78–84. 1997.

Hirai T., Taniura H., Goto Y., Tamaki K., Oikawa H., Kambe Y., Ogura M., Ohno Y., Takarada T., Yoneda Y. Counteraction by repetitive daily exposure to static magnetism against sustained blockade of N-methyl-D-aspartate receptor channels in cultured rat hippocampal neurons. J Neurosci Res. 80:491–500. 2005.

Invernizzi R., Bramante M., Samanin R. Extracellular concentrations of serotonin in the dorsal hippocampus after acute and chronic treatment with citalopram. Brain Res. 696:62–66. 1995.

Iwata M., Shirayama Y., Ishida H., Kawahara R. Hippocampal synapsin I, growth-associated protein-43, and microtubule-associated protein-2 immunoreactivity in learned helplessness rats and antidepressant-treated rats. Neuroscience. 141:1301–1313. 2006.

Kang M., Pyun KH., Jang CG., Kim H., Bae H., Shim I. Nelumbinis Semen reverses a decrease in hippocampal 5-HT release induced by chronic mild stress in rats. J Pharm Pharmacol. 57:651–656. 2005.

Kirby LG., Allen AR., Lucki I. Regional differences in the effects of forced swimming on extracellular levels of 5-hydroxytryptamine and 5-hydroxyindoleacetic acid. Brain Res. 682:189–196. 1995.

Kirby LG., Chou-Green JM., Davis K., Lucki I. The effects of different stressors on extracellular 5-hydroxytryptamine and 5-hydroxyindoleacetic acid. Brain Res. 760:218–230. 1997.

Korte-Bouws GA., Korte SM., De Kloet ER., Bohus B. Blockade of corticosterone synthesis reduces serotonin turnover in the dorsal hippocampus of the rat as measured by microdialysis. J Neuroendocrinol. 8:877–881. 1996.

Kuroda Y., McEwen BS. Effect of chronic restraint stress and tianeptine on growth factors, growth-associated protein-43 and microtubule-associated protein 2 mRNA expression in the rat hippocampus. Brain Res Mol Brain Res. 59:35–39. 1998.

Laifenfeld D., Karry R., Grauer E., Klein E., Ben-Shachar D. Antidepressants and prolonged stress in rats modulate CAM-L1, laminin, and pCREB, implicated in neuronal plasticity. Neurobiol Dis. 20:432–441. 2005.

Lamont SR., Paulls A., Stewart CA. Repeated electroconvulsive stimulation, but not antidepressant drugs, induces mossy fibre sprouting in the rat hippocampus. Brain Res. 893:53–58. 2001.

Larsen MH., Hay-Schmidt A., Ronn LC., Mikkelsen JD. Temporal expression of brain-derived neurotrophic factor (BDNF) mRNA in the rat hippocampus after treatment with selective and mixed monoaminergic antidepressants. Eur J Pharmacol. 578:114–122. 2008.

Manji HK., Moore GJ., Rajkowska G., Chen G. Neuroplasticity and cellular resilience in mood disorders. Mol Psychiatry. 5:578–593. 2000.

McGuire CB., Snipes GJ., Norden JJ. Light-microscopic immunolocalization of the growth- and plasticity-associated protein GAP-43 in the developing rat brain. Brain Res. 469:277–291. 1988.

Mennini T., Mocaer E., Garattini S. Tianeptine, a selective enhancer of serotonin uptake in rat brain. Naunyn Schmiedebergs Arch Pharmacol. 336:478–482. 1987.

Miyake K., Yamamoto W., Tadokoro M., Takagi N., Sasakawa K., Nitta A., Furukawa S., Takeo S. Alterations in hippocampal GAP-43, BDNF, and L1 following sustained cerebral ischemia. Brain Res. 935:24–31. 2002.

Mokler DJ., Torres OI., Galler JR., Morgane PJ. Stress-induced changes in extracellular dopamine and serotonin in the medial prefrontal cortex and dorsal hippocampus of prenatally malnourished rats. Brain Res. 1148:226–233. 2007.

Moreau JL., Jenck F., Martin JR., Mortas P., Haefely WE. Antidepressant treatment prevents chronic unpredictable mild stress-induced anhedonia as assessed by ventral tegmentum self-stimulation behavior in rats. Eur Neuropsychopharmacol. 2:43–49. 1992.

Naffah-Mazzacoratti MG., Funke MG., Sanabria ER., Cavalheiro EA. Growth-associated phosphoprotein expression is increased in the supragranular regions of the dentate gyrus following pilocarpine-induced seizures in rats. Neuroscience. 91:485–492. 1999.

Nestler EJ., Barrot M., DiLeone RJ., Eisch AJ., Gold SJ., Monteggia LM. Neurobiology of depression. Neuron. 34:13–25. 2002.

Ni YG., Gold SJ., Iredale PA., Terwilliger RZ., Duman RS., Nestler EJ. Region-specific regulation of RGS4 (Regulator of G-protein-signaling protein type 4) in brain by stress and glucocorticoids: in vivo and in vitro studies. J Neurosci. 19:3674–3680. 1999.
Papp M., Nalepa I., Antkiewicz-Michaluk L., Sanchez C. Behavioural and biochemical studies of citalopram and WAY 100635 in rat chronic mild stress model. Pharmacol Biochem Behav. 72:465–474. 2002.

Pawlak R., Magarinos AM., Melchor J., McEwen B., Strickland S. Tissue plasminogen activator in the amygdala is critical for stress-induced anxiety-like behavior. Nat Neurosci. 6:168–174. 2003.

Pawlak R., Rao BS., Melchor JP., Chattarji S., McEwen B., Strickland S. Tissue plasminogen activator and plasminogen mediate stress-induced decline of neuronal and cognitive functions in the mouse hippocampus. Proc Natl Acad Sci U S A. 102:18201–18206. 2005.

Paxinos G., Watson C. The Rat Brain in Stereotaxic Coordinates. 4th ed.Academic Press;San Diego: 1998.
Pittenger C., Duman RS. Stress, depression, and neuroplasticity: a convergence of mechanisms. Neuropsychopharmacology. 33:88–109. 2008.

Qin Y., Karst H., Joels M. Chronic unpredictable stress alters gene expression in rat single dentate granule cells. J Neurochem. 89:364–374. 2004.

Rosenbrock H., Koros E., Bloching A., Podhorna J., Borsini F. Effect of chronic intermittent restraint stress on hippocampal expression of marker proteins for synaptic plasticity and progenitor cell proliferation in rats. Brain Res. 1040:55–63. 2005.

Sairanen M., O'Leary OF., Knuuttila JE., Castren E. Chronic antidepressant treatment selectively increases expression of plasticity-related proteins in the hippocampus and medial prefrontal cortex of the rat. Neuroscience. 144:368–374. 2007.

Sandi C., Davies HA., Cordero MI., Rodriguez JJ., Popov VI., Stewart MG. Rapid reversal of stress induced loss of synapses in CA3 of rat hippocampus following water maze training. Eur J Neurosci. 17:2447–2456. 2003.

Shors TJ., Chua C., Falduto J. Sex differences and opposite effects of stress on dendritic spine density in the male versus female hippocampus. J Neurosci. 21:6292–6297. 2001.

Skene JH., Jacobson RD., Snipes GJ., McGuire CB., Norden JJ., Freeman JA. A protein induced during nerve growth (GAP-43) is a major component of growth-cone membranes. Science. 233:783–786. 1986.

Snipes GJ., Chan SY., McGuire CB., Costello BR., Norden JJ., Freeman JA., Routtenberg A. Evidence for the coidentification of GAP-43, a growth-associated protein, and F1, a plasticity-associated protein. J Neurosci. 7:4066–4075. 1987.

Sousa N., Lukoyanov NV., Madeira MD., Almeida OF., Paula-Barbosa MM. Reorganization of the morphology of hippocampal neurites and synapses after stress-induced damage correlates with behavioral improvement. Neuroscience. 97:253–266. 2000.

Stahl SM. Using secondary binding properties to select a not so selective serotonin reuptake inhibitor. J Clin Psychiatry. 59:642–643. 1998.

Stewart MG., Davies HA., Sandi C., Kraev IV., Rogachevsky VV., Peddie CJ., Rodriguez JJ., Cordero MI., Donohue HS., Gabbott PL., Popov VI. Stress suppresses and learning induces plasticity in CA3 of rat hippocampus: a three-dimensional ultrastructural study of thorny excrescences and their postsynaptic densities. Neuroscience. 131:43–54. 2005.

Storey JD., Robertson DA., Beattie JE., Reid IC., Mitchell SN., Balfour DJ. Behavioural and neurochemical responses evoked by repeated exposure to an elevated open platform. Behav Brain Res. 166:220–229. 2006.

Stout SC., Mortas P., Owens MJ., Nemeroff CB., Moreau J. Increased corticotropin-releasing factor concentrations in the bed nucleus of the stria terminalis of anhedonic rats. Eur J Pharmacol. 401:39–46. 2000.

Torres IL., Gamaro GD., Vasconcellos AP., Silveira R., Dalmaz C. Effects of chronic restraint stress on feeding behavior and on monoamine levels in different brain structures in rats. Neurochem Res. 27:519–525. 2002.
Go to : 
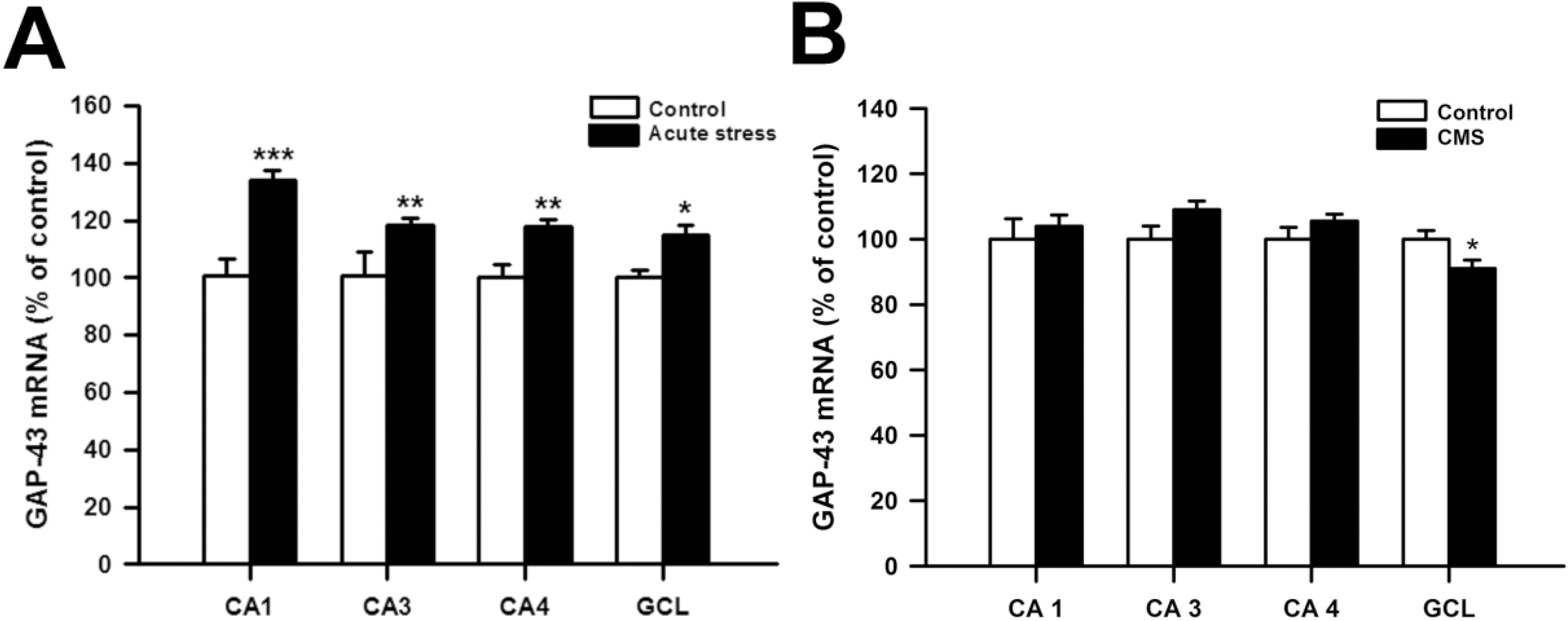 | Fig. 1.(A) The effects of acute stress on GAP-43 mRNA levels in CA1, CA3, CA4 and dentate granular cell layer (GCL) of the hippocampus (n=4 per group) (B) The effects of chronic mild stress (CMS) on GAP-43 mRNA levels in the CA1, CA3, CA4 and GCL of the hippocampus (n=7 per group). The results are expressed as percent of mean value from control group and are mean standard error of mean (S.E.M.). Data were compared by Student's t-test. ∗p<0.05, ∗∗p<0.01 and ∗∗∗p<0.001 vs. control group. |
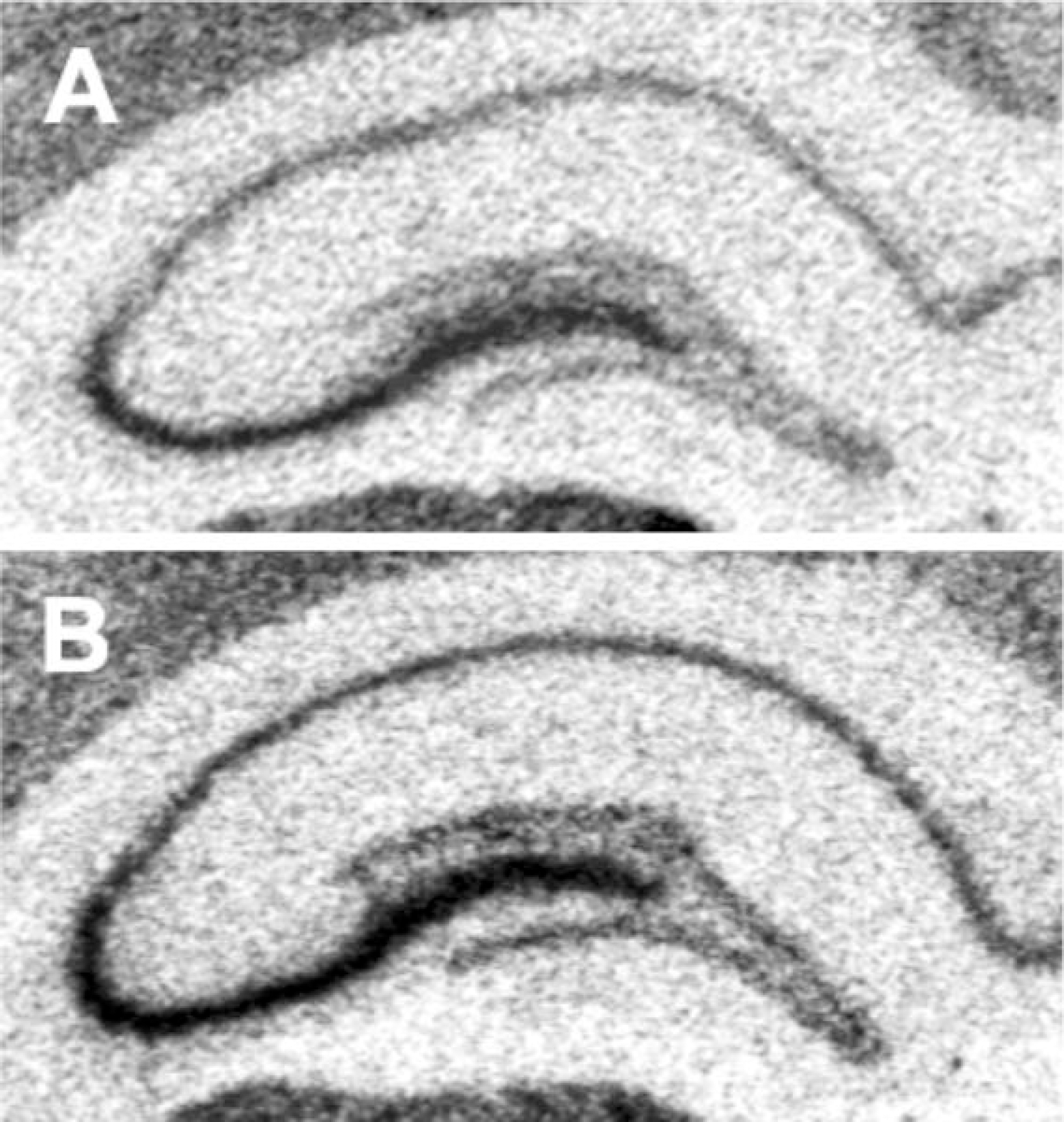 | Fig. 2.Representative autoradiographs of 35S-labeled GAP-43 mRNA expression in the hippocampus from each treatment group are shown. (A) control (B) acute immobilization stress. |
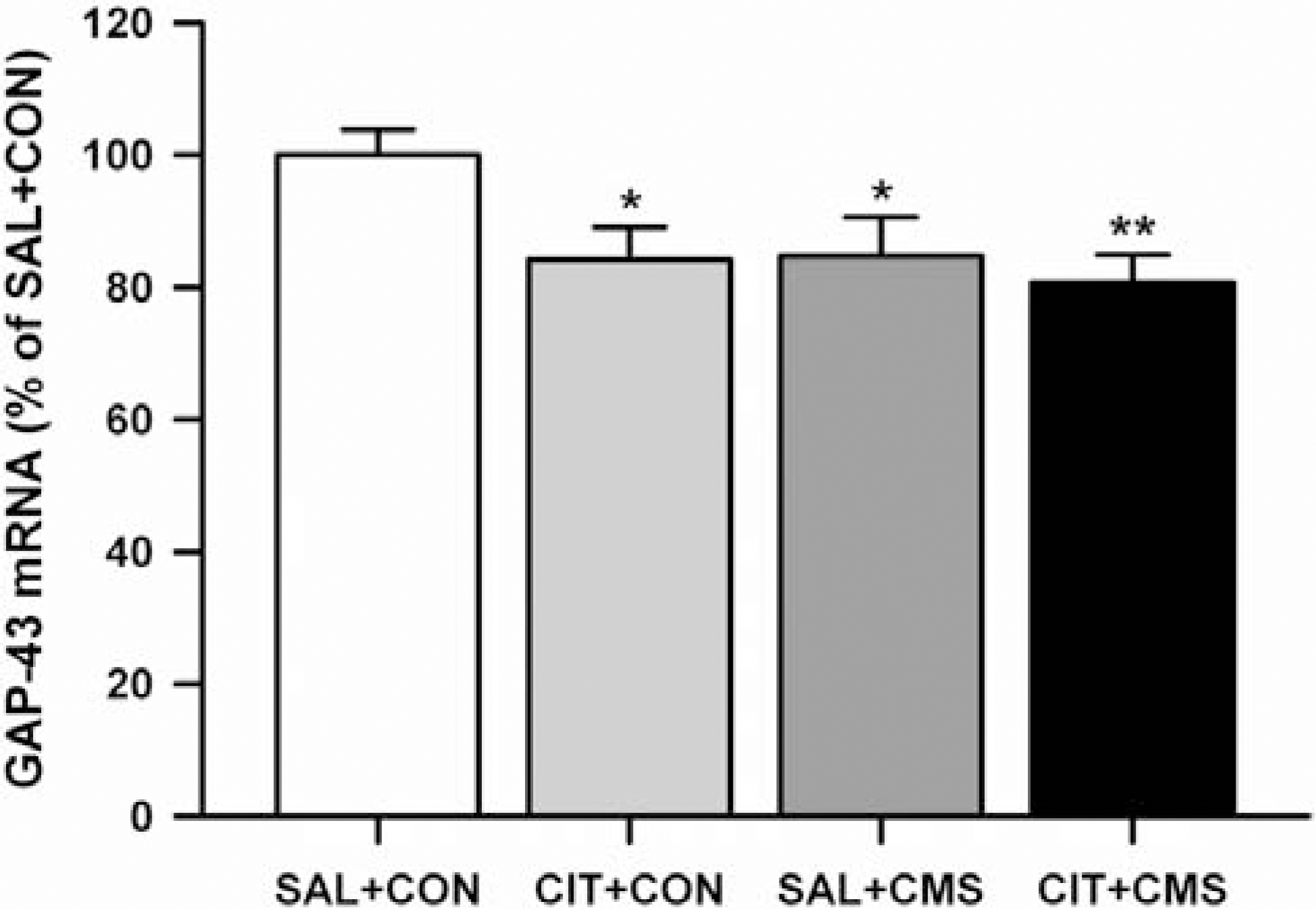 | Fig. 3.The effects of repeated citalopram treatment and CMS on GAP-43 mRNA levels in the GCL. Repeated citalopram treatment and CMS significantly decreased GAP-43 mRNA levels in the GCL, but there was no significant interaction between repeated citalopram treatment and CMS. The results are expressed as percent of mean value from SAL+CON group and are mean±S.E.M (n=9 per group). Data were compared using a one-way ANOVA with Fisher's LSD post hoc test. ∗p<0.05 and ∗∗p<0.01 vs. saline-treated unstressed control (SAL+CON). Abbreviations used: citalopram treated unstressed group (CON+CIT), saline treated CMS-exposed group (CMS+SAL), citalopram treated CMS-exposed group (CMS+CIT). |
 | Fig. 4.Representative autoradiographs of 35S-labeled GAP-43 mRNA in the hippocampus from each treatment group are shown. (A) saline treated unstressed group (B) citalopram treated unstressed group (C) saline treated CMS-exposed group (D) citalopram treated CMS-exposed group. |
Table 1.
Schedule of chronic mild stress
Table 2.
Effect of chronic mild stress and repeated citalopram treatment on the GAP-43 mRNAs levels in rat hippocampus
| CON+SAL | CON+CIT | CMS+SAL | CMS+CIT | |
|---|---|---|---|---|
| CA 1 | 100.0±7.6 | 96.5±3.0 | 107.4±3.0 | 88.1±7.3 |
| CA 3 | 100.0±4.3 | 97.4±1.1 | 106.1±2.6 | 86.3±4.8∗ |
| CA 4 | 100.0±3.4 | 99.8±1.6 | 105.7±2.4 | 86.1±4.8∗∗ |
The results are expressed as percent of mean value from SAL+CON group and are mean±S.E.M (n=9 per group). Data were compared using a one-way ANOVA with Fisher's LSD post hoc test. ∗p<0.05 and ∗∗p<0.01 vs. saline-treated unstressed control (SAL+CON). Abbreviations used are the same as those in Fig. 3.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download