Abstract
Ribbon-type antisense oligonucleotide to TGF-β1 (TGF-β1 RiAS) was designed and tested to prevent or resolve the fibrotic changes induced by CCl4 injection. When Hepa1c1c7 cells were transfected with TGF-β1 RiAS, the level of TGF-β1 mRNA was effectively reduced. TGF-β1 RiAS, mismatched RiAS, and normal saline were each injected to mice via tail veins. When examined for the biochemical effects on the liver, TGF-β1 mRNA levels were significantly reduced only in the TGF-β1 RiAS-treated group. The results of immunohistochemical studies showed that TGF-β1 RiAS prevented the accumulation of collagen and α-smooth muscle actin, but could not resolve established fibrosis. These results indicate that ribbon antisense to TGF-β1 with efficient uptake can effectively prevent fibrosis of the liver.
REFERENCES
Alcolado R., Arthur MJ., Iredale JP. Pathogenesis of liver fibrosis. Clin Sci (Lond). 92:103–112. 1997.

Arias M., Lahme B., Van de Leur E., Gressner AM., Weiskirchen R. Adenoviral delivery of an antisense RNA complementary to the 3’ coding sequence of transforming growth factor-beta1 inhibits fibrogenic activities of hepatic stellate cells. Cell Growth Differ. 13:265–273. 2002.
Arias M., Sauer-Lehnen S., Treptau J., Janoschek N., Theuerkauf I., Buettner R., Gressner AM., Weiskirchen R. Adenoviral expression of a transforming growth factor-beta1 antisense mRNA is effective in preventing liver fibrosis in bile-duct ligated rats. BMC Gastroenterol. 3:29. 2003.

Arthur MJ., Fibrogenesis II. Metalloproteinases and their inhibitors in liver fibrosis. Am J Physiol Gastrointest Liver Physiol. 279:G245–249. 2000.
Bajpai AK., Park JH., Moon IJ., Kang H., Lee YH., Doh KO., Suh SI., Chang BC., Park JG. Rapid blockade of telomerase activity and tumor cell growth by the DPL lipofection of ribbon antisense to hTR. Oncogene. 24:6492–6501. 2005.

Bharath MM., Chandra NR., Rao MRS. Prediction of an HMG-box fold in the C-terminal domain of histone H1: insights into its role in DNA condensation. Proteins. 49:71–81. 2002.

Bissell DM., Roulot D., George J. Transforming growth factor beta and the liver. Hepatology. 34:859–867. 2001.
Choi YK., Moon IJ., Jung HK., Jang BC., Seo SI., Park JG. Prevention of tissue injury by ribbon antisense to TGF-beta1 in the kidney. Int J Mol Med. 15:391–399. 2005.
Derossi D., Calvet S., Trembleau A., Brunissen A., Chassaing G., Prochiantz A. Cell internalization of the third helix of the Antennapedia homeodomain is receptor-independent. J Biol Chem. 271:18188–18193. 1996.

Dokka S., Toledo D., Shi X., Ye J., Rojanasakul Y. High-efficiency gene transfection of macrophages by lipoplexes. Int J Pharm. 206:97–104. 2000.

Eguchi A., Akuta T., Okuyama H., Senda T., Yokoi H., Inokuchi H., Fujita S., Hayakawa T., Takeda K., Hasegawa M., Nakanishi M. Protein transduction domain of HIV-1 Tat protein promotes efficient delivery of DNA into mammalian cells. J Biol Chem. 276:26204–26210. 2001.

Forbes SJ., Russo FP., Rey V., Burra P., Rugge M., Wright NA., Alison MR. A significant proportion of myofibroblasts are of bone marrow origin in human liver fibrosis. Gastroenterology. 126:955–963. 2004.

Friedman SL. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J Biol Chem. 275:2247–2250. 2000.

Friedman SL., Roll FJ., Boyles J., Bissell DM. Hepatic lipocytes: the principal collagen-producing cells of normal rat liver. Proc Natl Acad Sci U S A. 82:8681–8685. 1985.

Fulda S., Wick W., Weller M., Debatin KM. Smac agonists sensitize for Apo2L/TRAIL- or anticancer drug-induced apoptosis and induce regression of malignant glioma in vivo. Nat Med. 8:808–815. 2002.

Futaki S., Suzuki T., Ohashi W., Yagami T., Tanaka S., Ueda K., Sugiura Y. Arginine-rich peptides. An abundant source of membrane-permeable peptides having potential as carriers for intracellular protein delivery. J Biol Chem. 276:5836–5840. 2001.
George J., Roulot D., Koteliansky VE., Bissell DM. In vivo inhibition of rat stellate cell activation by soluble transforming growth factor beta type II receptor: a potential new therapy for hepatic fibrosis. Proc Natl Acad Sci U S A. 96:12719–12724. 1999.
Gressner AM., Weiskirchen R., Breitkopf K., Dooley S. Roles of TGF-beta in hepatic fibrosis. Front Biosci. 7:d793–807. 2002.

Gryaznov S., Skorski T., Cucco C., Nieborowska-Skorska M., Chiu CY., Lloyd D., Chen JK., Koziolkiewicz M., Calabretta B. Oligonucleotide N3'→P5’ phosphoramidates as antisense agents. Nucleic Acids Res. 24:1508–1514. 1996.

Hammel P., Couvelard A., O'Toole D., Ratouis A., Sauvanet A., Flejou JF., Degott C., Belghiti J., Bernades P., Valla D., Ruszniewski P., Levy P. Regression of liver fibrosis after biliary drainage in patients with chronic pancreatitis and stenosis of the common bile duct. N Engl J Med. 344:418–423. 2001.

Henry SP., Novotny W., Leeds J., Auletta C., Kornbrust DJ. Inhibition of coagulation by a phosphorothioate oligonucleotide. Antisense Nucleic Acid Drug Dev. 7:503–510. 1997.

Iredale JP., Benyon RC., Pickering J., McCullen M., Northrop M., Pawley S., Hovell C., Arthur MJ. Mechanisms of spontaneous resolution of rat liver fibrosis. Hepatic stellate cell apoptosis and reduced hepatic expression of metalloproteinase inhibitors. J Clin Invest. 102:538–549. 1998.

Issa R., Zhou X., Constandinou CM., Fallowfield J., Millward-Sadler H., Gaca MD., Sands E., Suliman I., Trim N., Knorr A., Arthur MJ., Benyon RC., Iredale JP. Spontaneous recovery from micronodular cirrhosis: evidence for incomplete resolution associated with matrix cross-linking. Gastroenterology. 126:1795–1808. 2004.

Kanzler S., Lohse AW., Keil A., Henninger J., Dienes HP., Schirmacher P., Rose-John S., zum Buschenfelde KH., Blessing M. TGF-beta1 in liver fibrosis: an inducible transgenic mouse model to study liver fibrogenesis. Am J Physiol. 276:G1059–1068. 1999.
Knittel T., Mehde M., Kobold D., Saile B., Dinter C., Ramadori G. Expression patterns of matrix metalloproteinases and their inhibitors in parenchymal and non-parenchymal cells of rat liver: regulation by TNF-alpha and TGF-beta1. J Hepatol. 30:48–60. 1999.
Ludtke JJ., Zhang G., Sebestyen MG., Wolff JA. A nuclear localization signal can enhance both the nuclear transport and expression of 1 kb DNA. J Cell Sci. 112:2033–2041. 1999.

Moon IJ., Choi K., Choi YK., Kim JE., Lee Y., Schreiber AD., Park JG. Potent growth inhibition of leukemic cells by novel ribbon-type antisense oligonucleotides to c-myb1. J Biol Chem. 275:4647–4653. 2000a.

Moon IJ., Kang H., Seu YB., Chang BC., Song DK., Park JG. Marked transfection enhancement by the DPL (DNA/peptide/lipid) complex. Int J Mol Med. 20:429–437. 2007.

Moon IJ., Lee Y., Kwak CS., Lee JH., Choi K., Schreiber AD., Park JG. Target site search and effective inhibition of leukaemic cell growth by a covalently closed multiple anti-sense oligonucleotide to c-myb. Biochem J. 346(Pt 2):295–303. 2000b.

Nakamura T., Ueno T., Sakamoto M., Sakata R., Torimura T., Hashimoto O., Ueno H., Sata M. Suppression of transforming growth factor-beta results in upregulation of transcription of regeneration factors after chronic liver injury. J Hepatol. 41:974–982. 2004.
Qi Z., Atsuchi N., Ooshima A., Takeshita A., Ueno H. Blockade of type beta transforming growth factor signaling prevents liver fibrosis and dysfunction in the rat. Proc Natl Acad Sci U S A. 96:2345–2349. 1999.
Schwarze SR., Ho A., Vocero-Akbani A., Dowdy SF. In vivo protein transduction: delivery of a biologically active protein into the mouse. Science. 285:1569–1572. 1999.
Sorgi FL., Bhattacharya S., Huang L. Protamine sulfate enhances lipid-mediated gene transfer. Gene Ther. 4:961–968. 1997.

Torchilin VP., Rammohan R., Weissig V., Levchenko TS. TAT peptide on the surface of liposomes affords their efficient intracellular delivery even at low temperature and in the presence of metabolic inhibitors. Proc Natl Acad Sci U S A. 98:8786–8791. 2001.

Ueno H., Sakamoto T., Nakamura T., Qi Z., Astuchi N., Takeshita A., Shimizu K., Ohashi H. A soluble transforming growth factor beta receptor expressed in muscle prevents liver fibrogenesis and dysfunction in rats. Hum Gene Ther. 11:33–42. 2000.

Vives E., Brodin P., Lebleu B. A truncated HIV-1 Tat protein basic domain rapidly translocates through the plasma membrane and accumulates in the cell nucleus. J Biol Chem. 272:16010–16017. 1997.
Fig. 1.
Experimental schedule. For 8 weeks of experiment, 1 ml/kg (body weight) of CCl4 was intraperitoneally administered twice per week. RiAS to mouse TGF-β1 or mismatched RiAS (100μg/30 g body weight) was also intravenously administered twice per week from the second week of experiment in the prevention group and mismatched group, and from the 5th week of experiment in the treatment group.

Fig. 2.
(A) Schematic representation of ribbon-type antisense to TGF-β1 (TGF-β1 RiAS). The stem is formed by complementary sequences at both ends of each oligo. The 5’ terminus of the stem has 4 bases of a single-stranded overhang of 5'-GATC-3'. Two TGF-β1 monomer molecules were ligated to generate a covalently closed molecule with diad symmetry. The RiAS oligos consist of two loops and an intervening stem. Each loop harbors an antisense sequence to TGF-β1. (B) Specific reduction of TGF-β1 mRNA by TGF-β1 RiAS. Hepa1c1c7 cells were transfected with DP complex and RT-PCR was conducted in order to determine the antisense activity of TGF-β1 RiAS. Transfection of TGF-β1 RiAS reduced TGF-β1 expression in Hepa1c1c7 cells. By way of contrast, however, when Hepa1c1c7 cells were treated with mismatched RiAS, TGF-β1 expression was not significantly affected. Mouse β-actin was as a control. Veh: vehicle, RiAS: TGF-β1 RiAS, MM: mismatched RiAS.
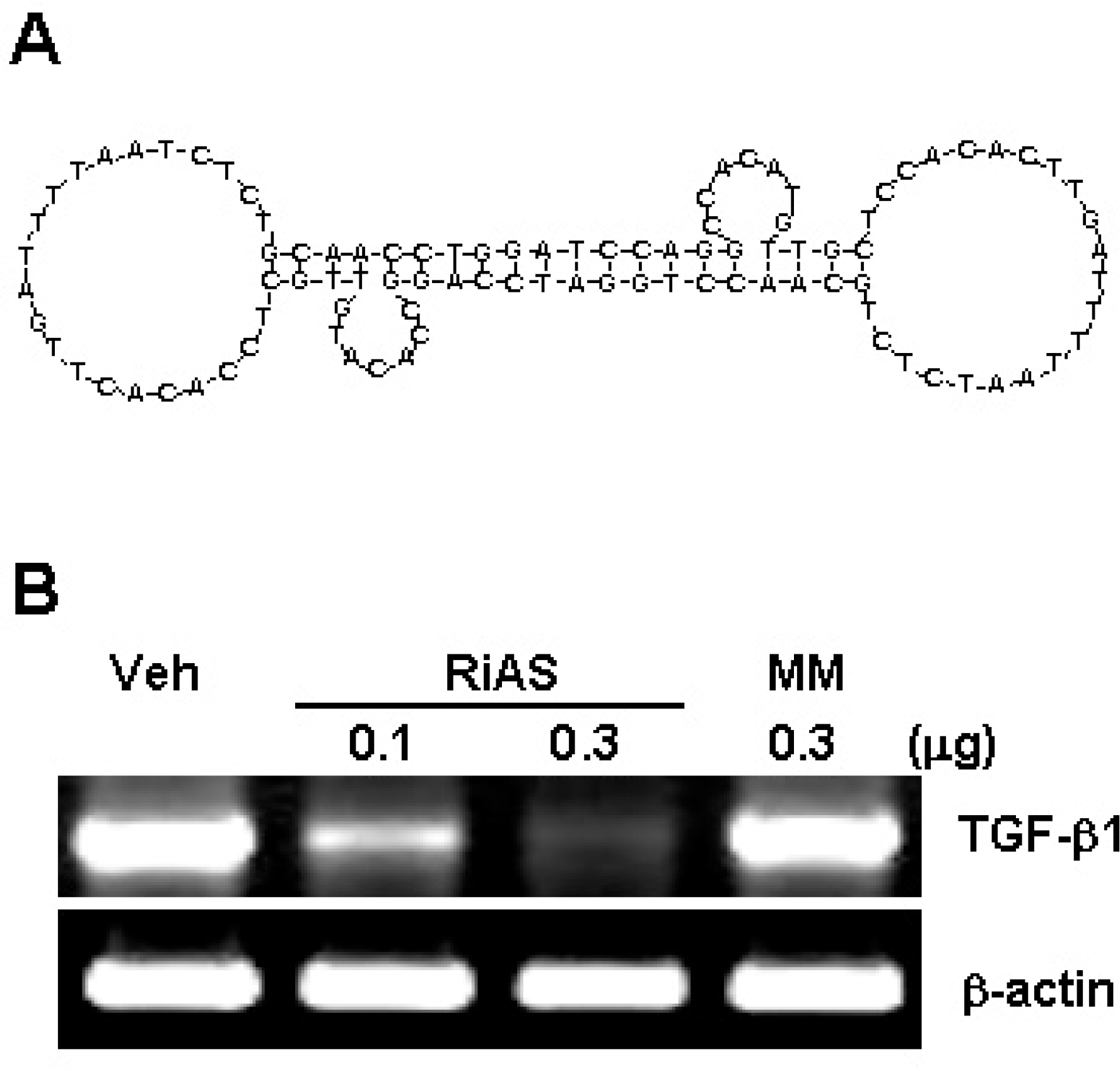
Fig. 3.
(A) The DP complex mediated transfection in Hepa1c1c7 mouse hepatoma cells. Transfection of FITC-labeled TGF-β1 RiAS was conducted using cationic peptide. The DP complex was added to Hepa1c1c7 cells for 24 hours. Fluorescence signals are shown in the right panel. (B) Normal saline (sham) or 10 μg of FITC-labeled TGF-β1 RiAS as a form of naked DNA only and DP complex were injected through the tail veins of normal or cirrhotic mice. Tissue sections of mice liver were observed under a fluorescence microscope (×200).

Fig. 4.
Histological observation of liver. Collagen deposition was detected as blue staining on Masson's trichrome staining (A). Immunohistochemistry for type I collagen (B) and α-smooth muscle actin (C). Staining was conducted with fixed and dehydrated tissues from mice treated with normal saline (CON), mismatched RiAS (MM), and TGF-β1 RiAS (Prevention and Treatment group). Stained tissues were mounted with a synthetic mounting solution for microscopic observation (×200).

Table 1.
Effect of TGF-β1 RiAS on serum biochemical parameters




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download