Accommodation is an increase in the refractive power of the lens of the eye to clearly see objects up close. The mechanism of accommodation is not fully understood. According to the commonly accepted Helmholtz theory, the ciliary muscle contracts during accommodation, pushing the ciliary body forward and relaxing the zonular fibers, while the thickness and convexity of the lens increase due to relaxation in the zonular fibers and the elastic nature of the lens capsule. Ultimately, the refractive power of the lens increases [
1]. The effect of this change during accommodation on the anterior chamber and the cornea has been evaluated by various measurement techniques in different populations [
23456789]. However, to the best of our knowledge, no study has been conducted on this subject in patients with refractive accommodative esotropia (RAE). Patients with moderate and severe hypermetropia accommodate more often than emmetropic patients to clear their blurred vision, and their squinting usually improves with eyeglasses or contact lenses [
10].
Materials and Methods
This prospective, nonrandomized, case-control study included 81 eyes of 81 patients who were divided into three groups as follows: the RAE group (31 eyes), the hypermetropia group (without strabismus, 25 eyes) and the emmetropia group (±0.50 diopters [D], 25 eyes). The hypermetropia and emmetropia groups were used as the control groups. The dominant squinting eye of the RAE patients and one randomly selected eye of the other groups were included in the study. The study was conducted in accordance with the Declaration of Helsinki and was approved by the ethical board of the Ulucanlar Research and Training Hospital. All patients and parents were informed about the details of this study and written consent of the parents was obtained before the study began. Since RAE typically occurs in pediatric patients, the study group mostly consisted of children. Patients who had undergone ocular surgery or were currently taking topical or systemic drugs that could affect accommodation and those with retina or optic nerve disorders or severe amblyopia (with a best-corrected distance visual acuity of less than 20 / 80) were excluded from the study.
After a complete ocular examination was performed, measurements were taken with the Pentacam HR. This device has a red light-emitting diode that serves as an active fixation target. The target can be moved from +2.0 to -5.0 D in 0.5-D steps, inducing physiological accommodation, so that anterior segment measurements can be obtained [
45]. The patients were instructed to look at the fixation target, and the measurements were taken from the eye-fixing target. All measurements were obtained by the same specialist, who had experience using the device under similar and scotopic conditions. Measurements were taken first in the non-accommodative condition (0.0 D) and then in the accommodative condition (-5.0 D). Pupil diameter (PD), anterior chamber depth (ACD), anterior chamber volume (ACV), and anterior chamber angle (ACA) were evaluated in four quadrants (0° / 90° / 180° / 270°) during each measurement session. The measurements of these parameters were automatically obtained by the Pentacam HR and then presented as a refractive map and cross-sectional Scheimpflug images. The angle of the intersection between the posterior corneal surface and the surface of the iris in the Scheimpflug image was used by the Pentacam HR to compute the ACA. Vertical (90° to 270°) and horizontal (0° to 180°) segments from 25 sections were used in the ACA evaluation. The distance between the endothelium and the anterior face of the crystalline lens at the corneal apex was used for the ACD. Once the Pentacam HR measurement had been obtained, cyclopentolate drops were administered three times at an interval of five minutes. A measurement was repeated with an autorefractometer (ARK-530A, Nidek, Gamagori, Japan) 45 minutes after the last drop was instilled.
Statistical analyses were performed using the IBM SPSS ver. 20 (IBM, A rmonk, NY, USA). A ll values were expressed as the mean ± standard deviation. The Shapiro-Wilk test was used to analyze data normality. Normality was not present, so all data and intragroup comparisons were conducted with the Wilcoxon signed-rank test, the intergroup comparisons with the Mann-Whitney U-test, and the comparison of three groups with the Kruskal-Wallis test. A p-value <0.05 was accepted as significant for the Wilcoxon and Mann-Whitney U-tests, and a value <0.0165 was considered significant for the Krusk
Results
Age, sex and refraction characteristics of the groups are presented in
Table 1. No difference was observed between the age and gender distributions of the groups (
p = 0.625 and
p = 482, respectively). The spherical equivalent was similar in the RAE and hypermetropia groups (
p = 0.842). The anterior chamber data of the groups regarding their non-accommodative and accommodative status and also the change with accommodation (Δ) together with the
p-value of the change are shown in
Table 2 and
Figs. 1,
2, to
3.
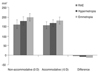
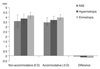
Non-accommodative status
The ACD, ACV, and PD values were 2.92 ± 0.29 mm, 162.7 ± 29.7 mm3, and 3.49 ± 0.79 mm in the RAE group, 3.10 ± 0.17 mm, 178.7 ± 16.7 mm3, and 3.74 ± 0.31 mm in the hypermetropia group and 3.21 ± 0.17 mm, 198.7 ± 17.5 mm3, and 4.05 ± 0.74 mm in the emmetropia group, respectively. When the three groups were compared, all parameters other than ACA were significantly different (ACD, p = 0.003; ACV, p = 0.009; PD, p = 0.002; 0°, p = 0.041; 90°, p = 0.068; 180°, p = 0.022; 270°, p = 0.025; respectively, Kruskal-Wallis test). ACD, ACV, and PD values in the RAE group were lower than those of both control groups (hypermetropia group: p = 0.006, p = 0.013, p = 0.024; emmetropia group: p = 0.003, p = 0.007, p = 0.003, respectively). The ACD, ACV and PD values for the hypermetropia group were lower than those obtained in the emmetropia group (p = 0.024, p = 0.022, and p = 0.011, respectively).
Accommodative status
The ACD, ACV and PD values were 2.89 ± 0.30 mm, 157.2 ± 28.6 mm3, and 3.47 ± 0.70 mm in the RAE group, 3.01 ± 0.24 mm, 168.9 ± 17.3 mm3, and 3.63 ± 0.41 mm in the hypermetropia group, and 3.07 ± 0.20 mm, 181.1 ± 18.2 mm3, and 3.89 ± 0.73 mm in the emmetropia group, respectively. When the three groups were compared, all parameters other than ACA were significantly different from each other (ACD, p = 0.005; ACV, p = 0.001; PD, p = 0.005; 0°, p = 0.084; 90°, p = 0.031; 180°, p = 0.044; 270°, p = 0.057; respectively, Kruskal-Wallis test). The ACD, ACV and PD values in the RAE group were lower than those of both control groups (hypermetropia group: p = 0.012, p = 0.024, p = 0.035; emmetropia group: p = 0.09, p = 0.011, and p = 0.006, respectively). The ACD was similar, while the ACV and PD were smaller in the hypermetropia group than the emmetropia group (p = 0.225, p = 0.034, and p = 0.026, respectively).
Non-accommodative – accommodative difference (Δ)
The ΔACD, ΔACV, and ΔPD values were -0.03 ± 0.07 mm, -5.5 ± 4.2 mm3, and -0.02 ± 0.07 mm in the RAE group, -0.09 ± 0.08 mm, -9.8 ± 7.0 mm3, and -0.11 ± 0.23 mm in the hypermetropia group, and -0.14 ± 0.04 mm, -13.6 ± 8.8 mm3, and -0.16 ± 0.24 mm in the emmetropia group, respectively. When the three groups were compared, parameters other than the ΔACA were significantly different (ΔACD, p = 0.003; ΔACV, p < 0.001; ΔPD, p = 0.006; 0°, p = 0.116; 90°, p = 0.053; 180°, p = 0.047; 270°, p = 0.032; respectively, Kruskal-Wallis test). None of the decreases were significant in the RAE group. The ΔACD, ΔACV and ΔPD were significant in the hypermetropia and emmetropia groups. ΔACD, ΔACV and ΔPD in the RAE group were significantly lower than both control groups (hypermetropia group: p = 0.004, p = 0.008, and p = 0.001; emmetropia group: p < 0.001, p = 0.001, p < 0.001, respectively). The ΔACD, ΔACV, and ΔPD were significantly lower in the hypermetropia group than in the emmetropia group.
Discussion
Dynamic changes occur in the ciliary body, lens and iris during accommodation; ultimately, the refraction of the lens increases [
1]. This rise may be associated with the forward movement of the lens or protrusion of the anterior pole of the lens [
47912]. These changes at the crystalline lens affect anterior segment biometry, especially ACD [
23456789]. The aim of our study was to evaluate the changes that take place during accommodation in the anterior segment biometric parameters of patients with RAE.
A comparison of studies in which biometric measurements of the anterior segment have been obtained is difficult. These studies have been carried out in various age/ethnic groups; as a result, different methods are used, and the measurements were obtained under different lighting conditions. These factors can affect the results. The parameter values other than PD in the emmetropia group of our study were similar to the results of another study conducted in a similar age group in a population with the same ethnic origin and using a Pentacam [
13]. However, the PD values from our study were lower. This difference is likely related to the lighting of the room in which the measurements were obtained, as they were assessed under scotopic status in our study but under dim light status in the previous study.
The ACD in hypermetropic patients has been shown to be lower than that of emmetropic patients in many previous studies [
1415]. We additionally observed that the ACV and PD were also lower while the ACA was similar. Our study has two other important findings. One is that RAE patients have lower ACD, ACV, and PD values than hypermetropic patients with similar refractive errors. Another important finding was that the ΔACD, ΔACV, and ΔPD values in patients with RAE were lower than those reported in hypermetropic patients. The changes that occur in anterior chamber biometry during accommodation have been studied many times with different measurement methods, and ACD and PD were found to decrease in various amounts in most of these studies [
9]. Yan et al. [
9] reported a 24-µ decrease of ACD in emmetropia and a 6-µ decrease in hypermetropia with every 1 D of accommodative stimulation using anterior segment optical coherence tomography. Dubbelman et al. [
16] used a Scheimpflug camera in young participants and reported a 38-µ decrease in ACD for every 1-D accommodation. We determined the ΔACD value to be 28 µ (140 µ/5 D) in emmetropic patients, 18 µ in hypermetropic participants and 6 µ in RAE patients.
Only a few studies have evaluated the change in ACV in light of accommodation. The ΔACV with a 5-D accommodation was found to be 4.12 mm
3 in young patients and 2.06 mm
3 in the elderly in a study of Ni et al. [
4]. In our study, the ACV in the non-accommodative state was highest in emmetropes but lowest in RAE patients. Similarly, the ΔACV was highest in emmetropes (13.6 mm
3), followed by hypermetropes (9.8 mm
3) and the RAE group (5.5 mm
3). However, Ni et al. [
4] evaluated Chinese patients and reported lower ΔACV and ACV values in the non-accommodative status compared with our patients. The number of studies on the ACA change with accommodation is limited. In a study [
17] where measurements were obtained using a Scheimpflug camera, there was a non-significant increase in ACA in young people; in another study [
18] that used ultrasonic biomicroscopy, a mild decrease was found in the ACA in pseudophakic patients during accommodation. Changes in ACA were minimal and not significant in all groups of our study.
The PD is known to decrease during accommodation due to synkinetic miosis [
9]. In a study conducted in emmetropes with a Scheimpflug camera, this decrease was 0.18 mm with a 4-D accommodation [
17]. The ΔPD values were 0.95 and 1.5 mm in studies conducted by Yan et al. [
9] and Baikoff et al. [
2], respectively, using anterior segment optical coherence tomography. The ΔPD was reported as 0.26 mm in hypermetropes [
9]. However, the measurements in these two studies were taken under dim light and with maximum accommodation. Our study was carried out using 5-D accommodation stimulation under scotopic conditions, and the ΔPD was 0.16 mm in emmetropes, 0.11 mm in hypermetropes and only 0.02 mm in the RAE group.
The lower ACD, ACV, and PD for a non-accommodative status and the low ΔACD, ΔACV, and ΔPD may be explained by the high accommodation effort present in a resting state in the RAE group. The lens thickness increased due to the high accommodative amplitude and accordingly may decrease t he ACD and ACV in t he RAE group while in a resting state. Since most of their accommodative capacity is used during rest, there may be less of a response to an accommodative stimulus in an accommodative state. Therefore, the ΔACD and ΔACV may be lower in RAE patients than in other groups. Because of synkinetic miosis during accommodation, PD may be smaller in the RAE group during a resting state. Similarly, the ΔPD may be lower because the accommodation capacity is used during a resting state. To obtain more definite data on this subject, future studies should assess the lens thickness in non-accommodative conditions, ACD after cycloplegia, and both the lens thickness and the ACD with a contact lens to correct the refractive error. Since the ciliary muscle relaxes with cycloplegia, the lens thickness and therefore the anterior chamber biometric values also change. This alteration is most marked in hypermetropes and least apparent in myopes [
19]. If further studies find that the lens thickness and the increase in ACD after cycloplegia in a non-accommodative state in RAE patients is higher than that of hypermetropic patients, it would further strengthen our findings. The measurements in our study were conducted without correcting refractive errors, which was similar to the method used in previous studies. It is important to obtain the measurements with a contact lens to correct the refractive error in RAE patients because the position of the crystalline lens in the non-accommodative and accommodative esotropia may change when the refractive errors of the patients are corrected.
The most important disadvantage of our study was its failure to correct the refractive error of patients during measurement. More valuable findings could be obtained if measurements were taken after refractive correction with contact lenses in these participants. The second important disadvantage of our study was that we did not evaluate the axial length of the patients. However, a relationship exists between the anterior chamber parameters and axial length [
20]. Another disadvantage of this study was that the mean ages of the groups were very low; however, the lens thickness and accommodation amplitude change with age [
2910]. Thus, alterations in the biometry of the anterior chamber in RAE patients may be different than those observed in emmetropic patients due to age. Further evaluation of older RAE patients may provide additional guidance on this subject.
In conclusion, the anterior chamber of RAE patients was shallower than that of patients with hypermetropia or emmetropia. However, there is a need for more studies to evaluate the biometric parameters after the correction of refractive error in older patients. These new studies may provide additional valuable information in RAE patients.







 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download