Abstract
Purpose
Interleukin (IL)-22 is a cytokine involved in epithelial cell regeneration. Currently, no research studies have analyzed the distribution of the three distinct IL-22–secreting cell populations in human or mouse conjunctiva. This study investigated the distribution of the three main populations of IL-22–secreting immune cells, αβ Th cells, γδ T cells, or innate cells (innate lymphoid cells [ILCs] or natural killer cells), in conjunctival associated lymphoid tissues (CALTs) in human and mouse models.
Methods
We collected discarded cadaveric bulbar conjunctival tissue specimens after preservation of the corneo-limbal tissue for keratoplasty from four enucleated eyes of the domestic donor. The bulbar conjunctiva tissue, including the cornea from normal (n = 27) or abraded (n = 4) B6 mice, were excised and pooled in RPMI 1640 media. After the lymphoid cells were gated in forward and side scattering, the αβ Th cells, γδ T cells, or innate lymphoid cells were positively or negatively gated using anti-CD3, anti-γδ TCR, and anti–IL-22 antibodies, with a FACSCanto flow cytometer.
Results
In normal human conjunctiva, the percentage and number of cells were highest in αβ Th cells, followed by γδ T cells and CD3– γδ TCR – IL-22+ innate cells (presumed ILCs, pILCs) (Kruskal-Wallis test, p = 0.012). In normal mice keratoconjunctiva, the percentage and total number were highest in γδ T cells, followed by αβ Th cells and pILCs (Kruskal-Wallis test, p = 0.0004); in corneal abraded mice, the population of αβ Th cells and pILCs tended to increase.
Interleukin (IL)-22 is an anti-inflammatory cytokine involved in epithelial cell regeneration [1,2]. The role of IL-22 is clearly revealed in gut tissue remodeling and facilitates balance between commensal bacteria and the host immune system [1]. IL-22 is mainly secreted from immune cells, such as CD4 αβ T cells (most notably Th22 cells), γδ T cells, natural killer (NK) cells, and innate lymphoid cells (ILCs) [1,2]. In the cornea, IL-22–secreting γδ T cells and NK cells contribute to the anti-inflammatory response and promote epithelial healing in mouse models [3456]. However, there are not currently any reports on the distribution of IL-22–secreting immune cells, including CD4 αβ T cells, γδ T cells, and IL-22–secreting innate cells in human conjunctival associated lymphoid tissues (CALTs). Recently, ILCs have been discovered in mucosal associated lymphoid tissues, including the intestinal tract, lungs, and skin. ILCs might play a role in epithelial cell homeostasis and in epithelial cell regeneration via secretion of IL-22 [78]. However, to date, no study has reported the presence of ILCs in the human or mouse eye.
We investigated the distribution of IL-22–secreting immune cells, including αβ T cells, γδ T cells, or innate cells (ILCs or NK cells), in CALTs of mouse and human models and assessed cell population distributions in CALTs.
All procedures used in this study strictly adhered to the Association of Research in Vision and Ophthalmology (ARVO) Statement regarding the use of animals in ophthalmic and vision research. The experimental protocol was approved by the Ethics Committee at Seoul National University Hospital Biomedical Research Institute (13-0093-C1A0 (1)). Seven to 10-week-old male B6 mice were used in this study. All mice were bred and maintained in the Mouse Facility at the Biomedical Research Institute of Seoul National University Hospital, in a specific pathogen- free environment, with free access to water and food. Twenty-seven mice were used for these experiments (23 for five normal condition experiments, four for a corneal abrasion model experiment).
This study was conducted in accordance with the guidelines of the Declaration of Helsinki and was approved by the institutional review board of Seoul National University Hospital (1102-092-353). We collected specimens of discarded cadaveric bulbar conjunctival tissue after preservation of the corneo-limbal tissue for keratoplasty from four enucleated eyes of domestic donors. Informed consent was obtained to use a part of discarded tissue for a laboratory testing. In the enucleation procedure, the bulbar conjunctiva was dissected 6 to 7 mm from the limbus. Corneo-limbal tissue, which included peripheral conjunctiva at least 2 to 3 mm from the limbus, was harvested from the eyeball for preservation in Optisol (Chiron Ophthalmics, Irvine, CA, USA). The remaining conjunctival tissue was preserved in RPMI 1640 media (10mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid + 10% fetal bovine serum + 1% penicillin-streptomycin; Welgene, Seoul, Korea) for as long as 12 hours or 7 days and stored at 4℃. Donor demographics and time factors that might affect outcome are described in Table 1.
To isolate conjunctival tissue from the human donor, preserved conjunctival tissues were cut into small pieces (1 cm × 1 cm) and pre-digested in pre-digestion solution (GIBCO Hank's balanced salt solution [HBSS] containing 5 mM ethylenediaminetetraacetic acid, and 1 mM dithiothreitol) at 37℃ for 20 minutes. The pre-digested tissue and suspended solution were passed through a 100-µm nylon cell strainer to collect the freed cells, and pre-digested tissue was incubated again in pre-digestion solution at 37℃ for another 20 minutes and then was passed through a 100-µm nylon cell strainer. After washing, the tissues were cut into small pieces and incubated in digestion solution (0.05 g of collagenase D [Roche, Indianapolis, IN, USA] + 0.05 g of DNase I [Roche] + 0.3 g of dispase II [Roche] in phosphate-buffered saline) at 37℃ for 20 minutes. The tissue solution was vortexed for 20 seconds, and the supernatant was passed through a 40-µm nylon cell strainer to collect freed cells. The collected supernatant was then centrifuged for 20 minutes at 500 g. This procedure was repeated twice for the remaining tissues. The cell pellet was suspended in 10 ml of 40% Percoll (1.129 g/mL; GE Healthcare, Little Chalfont, UK), layered onto 5 mL of 80% Percoll, and centrifuged (2,400 rpm, 4℃, 30 minutes). The cells between the layers were collected, washed in phosphate-buffered saline, and re-suspended in RPMI 1640 media.
To isolate the immune cells from mouse keratoconjunctival tissue, the eyes from three to eight 10-week-old B 6 mice were pooled in each experiment (n = 27). The bulbar conjunctiva tissue, including the cornea from B6 mice, was excised and collected in RPMI 1640 media. We modified the protocol for humans and did not include digestive enzyme, pre-digestive solution, or Percoll because mouse conjunctival tissue is more fragile than human tissue. Keratoconjunctival tissues were cut into small pieces and incubated in HBSS for 30 minutes at 37℃. The tissues were minced with a syringe plunger, reincubated in HBSS, vortexed for 20 seconds, and passed through a 40-µm nylon cell strainer. The supernatant was collected, centrifuged (1,800 rpm, 7℃, 10 minutes), and resuspended in RPMI 1640. This procedure of vortexing, filtering, and centrifuging was repeated six times with the remaining tissue.
To investigate the changes of IL-22–secreting cells, the cornea of the right eye was marked with 2 mm trephine, and the epithelial layer was removed with a surgical blade. Forty-eight hours later, the seven-week-old B6 mice (n = 4) were sacrificed, and bulbar conjunctiva including the cornea were harvested.
Cell suspensions were incubated for 30 minutes at 4℃ with fluorescein-conjugated anti-human or anti-mouse antibodies: CD3 and γδ TCR. For intracellular IL-22 staining, cells were stimulated for 5 hours with 10 ng/mL IL-1β and 10 ng/mL IL-23 in the presence of GolgiStop (BD Pharmingen, San Diego, CA, USA). Nonspecific staining was blocked by purified 2.4G2 Ab (BD Fc Block). The following combinations of color conjugation and gating strategies were used: (1) For human, anti-human CD3-FITC (1:20; eBioscience, San Diego, CA, USA) + anti-human γδ TCR-PerCP cy5.5 (1:20, eBioscience) + anti-human IL-22-PE (1:20, eBioscience), (2) For mouse, anti-mouse CD3-Per-CP cy5.5 (1:100, eBioscience) + anti-mouse γδ TCR-FITC (1:100, eBioscience) + anti-mouse IL-22-PE (1:100, eBioscience). CD3+ γδ TCR+IL-22+ cells were gated as γδ T cells, CD3+ γδ TCR-IL-22+ cells were gated as αβ Th cells (or presumed Th22 cells [pTh22 cells]), and CD3- γδ TCR-IL-22+ cells were gated as presumed ILCs (pILCs) after the less granular and smaller cells were gated in forward and side scattering as a lymphoid cell gating. The cells were assayed for fluorescence using a FACSCanto flow cytometer (BD BioSciences, Mountain View, CA, USA). Data was analyzed using the Flowjo program (Tree Star, Ashland, OR, USA).
GraphPad Prism ver. 6 (GraphPad Software, La Jolla, CA, USA) was used for statistical tests. Data was tested for a Gaussian distribution using the D'Agostino-Pearson omnibus test. To compare means of more than two groups, data was analyzed by the Kruskal-Wallis test. To compare means of two groups, data was analyzed by the Mann-Whitney test. The data are presented as the mean ± standard error. Differences were considered significant at p < 0.05.
In normal human conjunctiva, CD3+ γδ TCR − IL-22+ cells (αβ Th cells), CD3+ γδ TCR + IL-22+ cells (γδ T cells), and CD3− γδ TCR − IL-22+ cells (pILCs) were found. The percentage was highest in CD3+ γδ TCR − IL-22+ cells (αβ Th cells), followed by γδ T cells and CD3− γδ TCR − IL-22+ innate cells (Fig. 1A, 1B). The total number was also highest in αβ Th cells, followed by γδ T cells and pILCs, and these results were statistically significant (Kruskal-Wallis test, p = 0.012) (Fig. 1A, 1C). In normal mouse keratoconjunctiva, CD3+ γδ TCR − IL-22+ cells (αβ Th cells), γδ T cells, and CD3− γδ TCR − IL-22+ cells (pILCs) were observed. The percentage and total number were highest in γδ T cells, followed by αβ Th cells and pILCs (Kruskal-Wallis test, p = 0.0004 in percentage) (Fig. 2A-2C). The populations of αβ Th cells and pILCs were markedly increased (Fig. 2D, 2E) in corneal abraded mice.
IL-22 is expressed in various tissues, including gut, skin, lung, liver, and eyes. Many studies have reported that IL-22 mediates epithelial tissue wound healing for tissue protection and regeneration [9]. Recent studies have shown that the acinar cells of lacrimal glands secreted IL-22 in the dry eye mouse model, and IL-22 suppressed IL-17–mediated ocular mucosal inf lammation in dry eyes [10]. Chronic overexpression of IL-22 can lead to hyper-proliferation of epithelial cells and to increase of inflammatory signal especially in psoriasis. Therefore, IL-22 might play an important role in determining tissue or disease status [9]. In this study, we validated the presence of IL-22–secreting immune cells in human bulbar conjunctiva of cadaver donors, in which the main population was αβ T cells (pTh22 cells). We also found three populations of IL-22–secreting cells in the normal keratoconjunctival tissues of B6 mice, in which the main population was γδ T cells. Meanwhile, the proportions of αβ Th cells (pTh22 cells) and pILCs increased in abraded mice cornea, which indicates that αβ Th cells (pTh22 cells) and pILCs have a role in epithelial wound healing in the mouse model, as well as γδ T cells.
Distribution of IL-22 cells between the mouse and human models differs, presumably due to species-specific regulation. Another possibility for the differences in distribution is that the human tissue did not include the cornea in which intraepithelial γδ T cells reside, while the mouse tissue is known to have many γδ T cells, both in the cornea and the conjunctiva. We examined both species to determine whether distribution of IL-22–secreting immune cells in mouse CALT was representative of human CALT. We found that there were differences between the two models, which suggests that careful interpretation is needed when clinical relevance on the functional role of IL-22 is drawn from mouse experiments. Further human studies that investigate the role of IL-22 in the ocular surface are needed.
In human tissues, there are multiple factors that affect the number of isolated cells. These factors include age, preservation time (harvest-to-isolation), death-to harvest time, and the size of the collected conjunctiva; longer death-to-harvest time affects the number of freed cells. One of the critical issues was that we could not collect the same size of the conjunctiva from each donor, because the size of the conjunctival tissue was not always identical to the other collected samples. Despite this conjunctival size variation, the proportion of the IL-22–secreting immune cells (αβ Th cells > γδ T cells > pILCs) corresponded well with the percentage of IL-22–secreting immune cells (αβ Th cells > γδ T cells > pILCs). Therefore, we presumed that conjunctiva size variation did not have a significant effect on outcome.
The role of the γδ T cells is well known in corneal epithelial wound healing and epithelial homeostasis in the animal model [4511]. However, it is currently unknown whether IL-22–secreting immune cells have a critical role in the human eye. Therefore, we investigated the presence and distribution of IL-22–secreting immune cells in the human eye. Cells with lymphoid lineage primarily secrete IL-22, including αβ Th cells, γδ T cells, NK cells, NKT cells, and ILCs. In the human eye, αβ Th cells that produce IL-22 are Th1, Th17, and Th22 cells. It is presumed that 50% of αβ Th cells produce IL-22 alone (Th 22 cells), 33% of αβ Th cells co-produce IL-22 and IFNγ (Th1 cells), and 15% of αβ Th cells co-produce IL-22 a nd IL-17 (Th 17 cells), while IL-22 is mainly produced by Th17 cells in the mouse eye [9]. In addition, as a non-lymphoid lineage, a few macrophages can produce IL-22. However, macrophages can usually be eliminated in FSC/SSC lymphoid cell gating [12]. Thus, in the gating of CD3+ γδ TCR − IL-22+ cells, Th1, Th17, and NKT cells might be included, as well as Th22 cells. In the gating of CD3− γδ TCR − IL-22+cells, NK cells can be included, as well as ILCs. Discrimination of ILCs from NK cells is challenging, especially in tissue with scarce ILC. NKp44 in humans and NKp46 in mice, which are positive markers in ILCs, overlapped in both ILCs and NK cells, or some of the ILCs that secrete IL-22 did not express Nkp44 or Nkp46. Co-staining of CD45, CD127, or RORγt can be beneficial to differentiate ILCs from NK or other innate cells [13]. Nevertheless, our data suggest that αβ Th cells, which include Th22 (in some portion, Th1 and Th17), might have a role in ocular surface wound healing of the human eye, showing the largest distribution compared to γδ T cells or CD3− γδ TCR − IL-22+ innate cells (ILCs or NK cells). Further marker studies to fully characterize the subpopulation of IL-22–secreting cells are in progress. Another interesting finding is that the proportions of both αβ Th cells (CD3+ γδ TCR − IL-22+ cells) and pILCs (CD3− γδ TCR − IL-22+ cells) tended to increase in abraded cornea in the mouse eye, although this result was not statistically significant. This finding suggests that both αβ Th cells and innate cells (ILC3 or NK cells) play a role in epithelial wound healing, as well as γδ T cells. A clear role in each population should be validated in further studies.
There were some limitations to this study. Co-expressed IL-17A was not investigated. Considering that the pro-inflammatory versus tissue-protective functions of IL-22 can be regulated by the co-expressed cytokine IL-17A, a further experiment to examine co-expression of IL-17A should be performed. However, this descriptive study presents different distributions of three distinctive populations of IL-22–secreting immune cells (αβ Th cells, γδ T cells, innate cells) in human and mouse eye models. Finally, this is the first report of a human study that analyzed the presence of IL-22–secreting immune cells in the conjunctiva.
Figures and Tables
Fig. 1
Distribution of the interleukin (IL)-22–secreting cells in human conjunctiva. (A) A representative photo of the gating strategy for αβ Th cells (CD3+ γδ TCR − IL-22+), γδ T cells (CD3+ γδ TCR + IL-22+), and pILCs (CD3− γδ TCR − IL-22+). (B) The percentage was highest in αβ Th cells, followed by γδ T cells and pILCs. (C) The total number was highest in αβ Th cells, followed by γδ T cells and presumed innate lymphoid cells, which was statistically significant (Kruskal-Wallis test, p = 0.012). *p < 0.05.
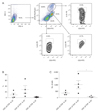
Fig. 2
Distribution of interleukin (IL)-22–secreting cells in mouse keratoconjunctiva. (A) A representative photo of gating strategy for αβ Th cells, γδ T cells, and presumed innate lymphoid cells (pILCs). (B) The percentage was highest in γδ T cells, followed by αβ Th cells and pILCs (Kruskal-Wallis test, p = 0.0004). (C) The number was highest in γδ T cells, followed by αβ Th cells and pILCs. (D,E) In corneal abraded mice, the populations of αβ Th cells and pILCs were markedly increased, although this result was not statistically significant. **p < 0.01.

Acknowledgements
This work was supported by a grant from the Seoul National University Hospital Research Fund (04-2013-0510).
Notes
References
1. Nikoopour E, Bellemore SM, Singh B. IL-22, cell regeneration and autoimmunity. Cytokine. 2015; 74:35–42.

3. Huang Y, Yang Z, Huang C, et al. γδ T cell-dependent regulatory T cells prevent the development of autoimmune keratitis. J Immunol. 2015; 195:5572–5581.

4. Li Z, Burns AR, Miller SB, Smith CW. CCL20, γδ T cells, and IL-22 in corneal epithelial healing. FASEB J. 2011; 25:2659–2668.

5. Li Z, Burns AR, Rumbaut RE, Smith CW. Gamma delta T cells are necessary for platelet and neutrophil accumulation in limbal vessels and efficient epithelial repair after corneal abrasion. Am J Pathol. 2007; 171:838–845.
6. Liu Q, Smith CW, Zhang W, et al. NK cells modulate the inflammatory response to corneal epithelial abrasion and thereby support wound healing. Am J Pathol. 2012; 181:452–462.

7. Sedda S, Marafini I, Figliuzzi MM, et al. An overview of the role of innate lymphoid cells in gut infections and inflammation. Mediators Inflamm. 2014; 2014:235460.

8. Goto Y, Obata T, Kunisawa J, et al. Innate lymphoid cells regulate intestinal epithelial cell glycosylation. Science. 2014; 345:1254009.

9. Dudakov JA, Hanash AM, van den Brink MR. Interleukin-22: immunobiology and pathology. Annu Rev Immunol. 2015; 33:747–785.

10. Ji YW, Mittal SK, Hwang HS, et al. Lacrimal gland-derived IL-22 regulates IL-17-mediated ocular mucosal inflammation. Mucosal Immunol. 2017; 10:1202–1210.

11. O'Brien RL, Taylor MA, Hartley J, et al. Protective role of gammadelta T cells in spontaneous ocular inflammation. Invest Ophthalmol Vis Sci. 2009; 50:3266–3274.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download