Abstract
Purpose
To assess long-term changes in intraocular pressure (IOP) and the development of glaucoma after early phacoemulsification in acute primary angle closure.
Methods
Retrospective chart review of acute primary angle closure patients treated with phacoemulsification in attack eyes versus fellow eyes. Within a month after the angle closure attack, all subjects underwent cataract surgery and were divided into two groups: group A received cataract surgery on their attack eyes. Group B also received cataract surgery on their fellow eye after phacoemulsification of the attack eyes. Study outcomes were the prevalence of IOP rise (occurrence of IOP >21 mmHg) and the incidence of newly developed glaucoma.
Results
Eighty-nine eyes were included, with 62 attack eyes in group A and 27 fellow eyes in group B. Group A (14 eyes, 22.58%) had a higher cumulative rate of IOP rise than group B (3 eyes, 11.11%) at 12 months (p = 0.001). Newly developed glaucoma was not observed in group B; however, 6 patients in group A developed glaucoma during the 12-month follow-up period (p < 0.001).
Conclusions
The attack eyes treated with phacoemulsification showed a significantly higher prevalence of IOP rise and newly developed glaucoma than fellow eyes that received phacoemulsification. These findings suggest that there is a possibility of IOP rise and development of glaucoma even when angle closure and successful IOP control have apparently been achieved after phacoemulsification.
Go to : 
Acute primary angle closure (APAC) is a sudden intraocular pressure (IOP) increase caused by abrupt occlusion of the drainage angle due to an exaggerated pupillary block. There is a high incidence of APAC in populations of East Asian origin [1]. The standard treatment modalities for APAC include the use of IOP-lowering medications and relief of pupillary block by laser peripheral iridotomy (LPI) [23]. Meanwhile, the lens plays an important role in the pathogenesis of APAC. The lens may narrow the angle by pushing the peripheral iris forward, and this effect will be more significant if the lens is affected by cataracts [45]. Hence, lens extraction in acute angle closure has had promising results and may result in less long-term peripheral anterior synechia (PAS) formation [67].
However, not all APAC cases treated with lens extraction have stable long-term outcomes. Husain et al. [8], prospectively studied 19 Singaporean patients treated with phacoemulsification or LPI in APAC. With failure defined as either an IOP between 22.0 and 24.0 mmHg or IOP of 25.0 mmHg during follow-up, the Kaplan-Meier survival estimate for success was about 90% over 2 years. Despite uncomplicated lens extraction, six patients (32%) required IOP-lowering medications, of which 2 (11%) were considered failures because of high IOP.
In this study, we conducted a retrospective chart review of APAC patients treated with early lens extraction by phacoemulsification. This study was performed to ascertain whether there are differences in the IOP rise and development of glaucoma between attack eyes and fellow eyes after phacoemulsification. We examined the hypothesis that long-term IOP control after phacoemulsification in attack eyes is not always successful because severe IOP elevation during an angle closure attack can induce trabecular meshwork damage.
This study was conducted at a university-based tertiary eye center. Patients were retrospectively recruited between October 2010 to November 2015. The study followed the declaration of Helsinki. Ethical approval was obtained from the institutional review board/ethics committee of the Hallym University Sacred Heart Hospital (2016-I131) and the informed consent was waived. and the informed consent was waived. This retrospective chart review included patients with a diagnosis of APAC who underwent cataract surgery. All patients had undergone a previous LPI in their attack eyes within days after the angle closure attack and had prophylactic LPI in their fellow eye. Within a month after the angle closure attack, all subjects received lens extraction by phacoemulsification with intraocular lens implantation. We divided the study subjects into two groups. Group A received cataract surgery on their attack eye, while group B also received cataract surgery on the fellow eye after lens extraction from attack eyes. All patients completed a minimum of 12 months of post-intervention follow-up.
The following criteria were used to define APAC cases [9]: (1) presence of at least two of the following symptoms: ocular or periocular pain, nausea/vomiting, or both, or an antecedent history of intermittent blurring of vision with haloes; (2) presenting IOP of more than 21 mmHg (as measured by Goldmann applanation tonometry) and the presence of at least three of the following signs: conjunctival injection, corneal epithelial edema, mid-dilated unreactive pupil, and shallow anterior chamber; and (3) the presence of an occluded angle in the affected eye, verified by gonioscopy.
The exclusion criteria [10] were (1) definite PAS formation over 1 quarter of a degree observed before and after lens extraction; (2) preoperatively diagnosed chronic angle closure glaucoma (CACG); (3) glaucomatous damage observed before and immediately after lens extraction; (4) ophthalmic disease that could affect IOP, other than cataracts; (5) any other intraocular surgery; and (6) secondary angle closure, such as lens-induced glaucoma, neovascular glaucoma, or uveitis. CACG eyes excluded from this study had a chronic IOP elevation over 21 mmHg (prior to treatment) along with glaucomatous optic neuropathy, corresponding visual field defects, and iridotrabecular contact over 3 quadrants on gonioscopy, along with a variable amount of PAS.
The primary outcome was the prevalence of IOP rise, which was defined as IOP >21 mmHg after weaning off glaucoma-related medication at 12 months and the prevalence of newly developed glaucoma. Secondary outcomes included mean IOP at each follow-up period and glaucoma medications required to maintain IOP ≤21 mmHg.
Data were collected on visual acuity (VA), IOP, number of topical and oral glaucoma medications, and preoperative gonioscopic examination at 1, 3, 6, and 12 months postoperatively. The preoperative and postoperative IOP values were derived from a mean of 2 IOP readings on 2 separate days using the Goldmann applanation tonometer. During every follow-up exam, IOP measurements and gonioscopic assessment of the drainage angle were made by a single trained glaucoma specialist and included a record of the extent of PAS formation in degrees of circumference, and the iridotrabecular angle width. The latter was estimated as the angle in degrees between a tangent to the surface of the trabecular meshwork and a tangent to the peripheral third of the iris, recorded using the Shaffer grading system and expressed as the mode of all 4 quadrants. Dynamic gonioscopy was used to detect the presence of PAS unless the angle was clearly wide open. PAS was defined as abnormal adhesion of the iris to the angle at least half a clock hour wide. VA was measured using the Snellen chart. Cataract grade were assessed by the Lens Opacities Classification System III.
A test for glaucoma was commonly performed within 1 to 2 months after cataract surgery. After that, the patient underwent regular visual field testing and optic nerve examination (disc photo, red-free retinal nerve fiber layer photo, and optical coherence tomography) at 4- to 6-month intervals according to the date of the outpatient visit.
All lens extraction surgery used phacoemulsification plus intraocular lens implantation performed by one surgeon. Under topical anesthesia, a 3.0-mm clear corneal incision was made. After standard phacoemulsification was performed, a posterior chamber intraocular lens with a 6.0-mm optic (intraocular lens; I-Flex, I-Medical Ophthalmic International Heidelberg, Heidelberg, Germany) was implanted. Because most eyes had mild or worse lens opacity, cataract surgery was performed to improve VA. However, when IOP was controlled poorly despite maximal topical or systematic medications, the eyes with a clear lens were treated with cataract surgery.
All analyses were performed using SPSS ver. 13.0 (SPSS Inc., Chicago, IL, USA). Data are expressed as mean values ± standard deviation of the mean and categorical variables were expressed as individual counts and proportions. Differences between the two groups were analyzed statistically using the independent samples t-test, Mann-Whitney U-test, Pearson's chi-square test, and the paired t-test. A Cox proportional hazard model was constructed to determine the significance of various predictors for IOP rise. A probability value of 0.05 was considered statistically significant for all analyses.
Go to : 
Of 67 consecutive patients, three were excluded due to a PAS over 1 quarter after phacoemulsification and two patients were excluded due to lens subluxation. Therefore, 62 eyes of 62 Korean subjects were included; 62 attack eyes in 62 patients made up group A and 27 fellow eyes in 27 patients made up group B. Patients in the two treatment groups were comparable in terms of most baseline characteristics. Baseline demographics and presenting clinical features are shown in Table 1. There were no significant differences between the groups in age, gender, laterality, peripheral anterior chamber width (Shaffer gonioscopic grading), and axial length. As expected, worse VA, higher IOP at presentation, and more myopic refractive values were observed in group A (p < 0.001 for all). In addition, the mean period between the attack and lens extraction procedures was shorter for attack eyes than for fellow eyes (p = 0.000). Table 2 shows the change in mean IOP levels over time in attack eyes versus fellow eyes. There were no statistically significant differences from one week after lens extraction to the 12-month follow-up visit.
Contrary to the mean IOP distribution, there was a notable difference in the prevalence of IOP rise (IOP >21 mmHg) between the two groups. The number of eyes that showed an IOP rise in group A and group B, respectively, was 5 versus 2 at 1 month, 3 versus 1 at 3 months, 3 versus 0 at 6 months, and 2 versus 0 at 12 months (Table 3). The cumulative rates of IOP rise in groups A and B were 8.06% versus 7.40% (p = 0.709) at 1 month, 12.90% versus 11.11% (p = 0.320) at 3 months, 17.74% versus 11.11% (p = 0.085) at 6 months, and 22.58% versus 11.11% (p = 0.001) at 12 months. The attack eyes had a prevalence of IOP rise (14 eyes, 22.58%) that was twice as high as the fellow eyes group (3 eyes, 11.11%) (p = 0.001) at 12 months. In other words, the cumulative probability of success 12 months after surgery was 88.89% in the fellow eyes group and 77.42% in the attack eyes group.
In addition, baseline demographics and clinical features upon presentation were assessed for potential associations with IOP rise. The results are summarized in Table 4. No significant differences were detected with regard to age, gender, time between LPI and lens extraction, VA, gonioscopy, spherical equivalent, or lens opacity. Only maximum IOP at the time of presentation was statistically associated with IOP rise (p = 0.025). A high IOP during the angle attack correlated with success of long-term IOP control.
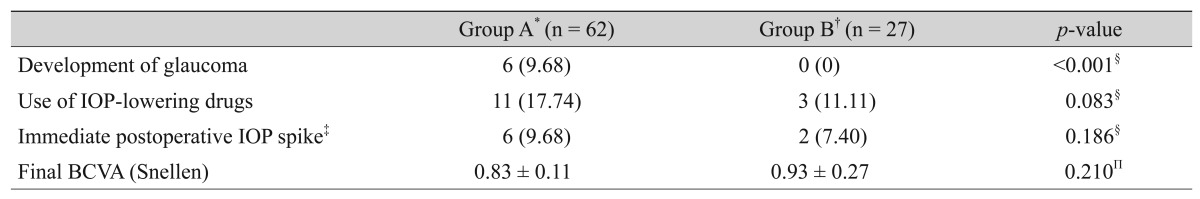
As another main outcome of our study, we investigated the incidence of newly developed glaucoma, defined as glaucomatous changes detected in the optic disc and corresponding visual field defects after postoperative follow-ups. As mentioned, we excluded patients with detected glaucoma with definite PAS formation over 1 quarter of a degree and only included open angle status, as confirmed by compressive goniosopy. In the present study, no patient in the fellow eyes group exhibited newly developed glaucoma during the 12-month follow-up; however, 6 patients in the attack eyes group did, and the difference between the two groups was significant (p < 0.001) (Table 5). The incidence of glaucoma was 1 patient within 1 year, 4 patients between 1 and 2 years, and 1 patient between 2 and 3 years after cataract surgery.
Group A (11 patients, 17.74%) had fewer patients than group B (3 patients, 11.11%) who used glaucoma medications to maintain IOP <21 mmHg, but this was not statistically significant (p = 0.083). An immediate pressure spike (IOP >25 mmHg) in the postoperative period occurred in 9.68% of eyes in group A and 7.40% of eyes in group B, which was not statistically significant (p = 0.186). Final VA did not differ significantly between the two groups (p = 0.210) (Table 5). Intraoperative and postoperative complications were few and manageable in both groups.
Go to : 
It has been reported that cataract surgery alone decreases IOP to some extent [1112]. The degree of IOP reduction differs depending on the type of glaucoma, and many studies have shown that phacoemulsification can improve IOP control in eyes with APAC [45678]. However, APAC patients treated with phacoemulsification do not always exhibit a decrease in IOP. Lai et al. [13] studied 21 patients with primary angle-closure glaucoma and co-existing visually significant cataracts in a prospective study. The study reported that 1 eye (4.8%) showed the same final and baseline IOP, while 5 eyes (23.8%) had a final IOP higher than the preoperative baseline. In the present study, the mean IOP decreased from 14.76 mmHg before lens extraction to 13.56 mmHg at 12 months in the attack eyes group. In the fellow eyes group, the mean IOP before lens extraction was 14.43 mmHg and it decreased to 13.34 mmHg at 12 months (Table 2). However, APAC treated with lens extraction does not always achieve long-term IOP control. Successful IOP control was achieved in 77.42% of eyes that had phacoemulsification in group A, compared with 88.89% in group B at the 12-month follow-up (Table 3). Moreover, the attack eye group had a higher likelihood of developing glaucoma. Newly developed glaucoma was not observed in the fellow eyes group, whereas 6 patients in the attack eyes group developed glaucoma during the follow up period (p = 0.000) (Table 5).
After an acute attack of angle closure glaucoma, some eyes continue to exhibit a raised IOP, despite a reversal of iridocorneal apposition and an open angle state. We cannot explain the specific reason for this result, but can hypothesize based on previous studies. Studies of the histopathology of the trabecular meshwork in primary angle closure glaucoma (PACG) described reduced or obliterated intertrabecular spaces and a considerable narrowing of Schlemm's canal [14]. Sihota et al. [15] studied trabeculectomy specimens using both light and electron microscopy in order to identify histopathological changes in the trabecular meshwork of eyes with acute PACG. All the acute PACG patients had an IOP of 54 to 62 mmHg, and had a 2-to 4-day duration of symptoms prior to surgery. In the study, the trabecular meshwork of acute PACG eyes revealed evidence of acute pigment release and a non-inflammatory degeneration of the endothelial cells and trabecular tissue. Because of trabecular damage from the angle-closure attack, it is possible that long-term IOP control was not achieved in APAC despite cataract surgery. Specifically, in the angle closure attack, a preexisting compromised trabecular outflow facility from repeated subacute angle closure attacks may be further impaired by released pigment granules and iris tissue fragments during the angle closure attack. It is because of this angle closure-induced mechanical obstruction of trabecular outflow channels that development of glaucoma may be observed, even though the angle is open after lens extraction. Another study reported the presence of extensive trabecular changes in acute PACG, even in areas without PAS. Prior iridotrabecular contact could also leave residual iris tissue attached to the trabecular surface [1617]. This may not be visible on gonioscopy. Therefore, gonioscopic evaluation of the extent of PAS may not truly reflect the extent of trabecular meshwork damage in acute PACG. Another possible etiology is that reactive trabecular changes after such an event would amplify ongoing age-related changes in the trabecular meshwork and could lead to uncontrolled IOP in the future [18].
Interestingly, although their trabecular meshwork is compromised, the patients who experienced a subsequent IOP rise also showed an IOP reduction in the early phase after cataract surgery in the attack eyes group. Although the exact mechanisms of this IOP decrease in the early period after lens extraction remain unclear, it has been shown that aqueous outflow facility is transiently accelerated after phacoemulsification [19]. In the current study, IOP reduction was observed in not only the fellow eyes group, but also the attack eyes group. Specifically, glaucomatous eyes also exhibited a postoperative IOP decrease. However, this effect may be transient because the trabecular meshwork is damaged during angle closure attacks. Because some eyes that develop an increase in IOP do so within the first 12 months of presentation, close monitoring of IOP and the optic disc are advised during the follow-up period in APAC patients treated with phacoemulsification.
When APAC eyes experience a sudden rise in IOP, the effect on the trabecular meshwork is variable because the severity of IOP elevation, duration of the attack, age, and previous function of the trabecular meshwork are different. In previous studies, high IOP upon presentation or before surgery was associated with a higher likelihood of IOP failure after phacoemulsification by Kaplan-Meier analysis in PACG patients [1220]. In the current study, the initial IOP in the group that subsequently experienced an IOP rise was significantly higher than in those patients for whom IOP was successfully controlled (Table 4). This implies that preoperative IOP status is a predictor of successful postoperative IOP control. This also suggests that prompt IOP reduction is needed upon presentation in angle closure.
We acknowledge the limitations resulting from our retrospective study design. Patient selection for the study was biased in favor of those with complete medical records. The study examined clinical outcomes at 12 months after cataract extraction to allow for comparison at a specific time point; however, these results may not reflect the long-term outcomes of surgery in all eyes. Another limitation of the current study was that some eyes with pre-existing PAS of over 1 quarter degree before phacoemulsification might have been included because of an inadequate gonioscopy exam. In addition, the PAS may have been broken, either because of positive pressure created by viscoelastic material and fluid during surgery or as a result of lens extraction [12]. Therefore, the current study could have underestimated the presence of pre-existing PAS and may have included CACG patients at baseline.
Our experience shows that attack eyes treated with lens extraction had a significantly higher rate of IOP rise than fellow eyes. Moreover, the number of new cases of glaucoma was greater in the attack eyes group. Hence, clinicians should consider the possibility of IOP rise and the development of glaucoma in patients who undergo phacoemulsification, even when angle closure and IOP control appear to be successful.
Go to : 
References
1. Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006; 90:262–267. PMID: 16488940.

2. Robin AL, Pollack IP. Argon laser peripheral iridotomies in the treatment of primary angle closure glaucoma: long-term follow-up. Arch Ophthalmol. 1982; 100:919–923. PMID: 7092629.
3. Nolan WP, Foster PJ, Devereux JG, et al. YAG laser iridotomy treatment for primary angle closure in east Asian eyes. Br J Ophthalmol. 2000; 84:1255–1259. PMID: 11049950.

4. Hayashi K, Hayashi H, Nakao F, Hayashi F. Changes in anterior chamber angle width and depth after intraocular lens implantation in eyes with glaucoma. Ophthalmology. 2000; 107:698–703. PMID: 10768331.

5. Melese E, Peterson JR, Feldman RM, et al. Comparing laser peripheral iridotomy to cataract extraction in narrow angle eyes using anterior segment optical coherence tomography. PLoS One. 2016; 11:e0162283. PMID: 27606482.

6. Shams PN, Foster PJ. Clinical outcomes after lens extraction for visually significant cataract in eyes with primary angle closure. J Glaucoma. 2012; 21:545–550. PMID: 21623222.

7. Azuara-Blanco A, Burr J, Ramsay C, et al. Effectiveness of early lens extraction for the treatment of primary angle-closure glaucoma (EAGLE): a randomised controlled trial. Lancet. 2016; 388:1389–1397. PMID: 27707497.

8. Husain R, Gazzard G, Aung T, et al. Initial management of acute primary angle closure: a randomized trial comparing phacoemulsification with laser peripheral iridotomy. Ophthalmology. 2012; 119:2274–2281. PMID: 22885123.
9. Foster PJ, Buhrmann R, Quigley HA, Johnson GJ. The definition and classification of glaucoma in prevalence surveys. Br J Ophthalmol. 2002; 86:238–242. PMID: 11815354.

10. Moghimi S, Vahedian Z, Fakhraie G, et al. Ocular biometry in the subtypes of angle closure: an anterior segment optical coherence tomography study. Am J Ophthalmol. 2013; 155:664–673. PMID: 23246271.

11. Nonaka A, Kondo T, Kikuchi M, et al. Angle widening and alteration of ciliary process configuration after cataract surgery for primary angle closure. Ophthalmology. 2006; 113:437–441. PMID: 16513457.

12. Hayashi K, Hayashi H, Nakao F, Hayashi F. Effect of cataract surgery on intraocular pressure control in glaucoma patients. J Cataract Refract Surg. 2001; 27:1779–1786. PMID: 11709251.

13. Lai JS, Tham CC, Chan JC. The clinical outcomes of cataract extraction by phacoemulsification in eyes with primary angle-closure glaucoma (PACG) and co-existing cataract: a prospective case series. J Glaucoma. 2006; 15:47–52. PMID: 16378018.

14. Rodrigues MM, Spaeth GL, Sivalingam E, Weinreb S. Histopathology of 150 trabeculectomy specimens in glaucoma. Trans Ophthalmol Soc U K. 1976; 96:245–255. PMID: 1070878.
15. Sihota R, Lakshmaiah NC, Walia KB, et al. The trabecular meshwork in acute and chronic angle closure glaucoma. Indian J Ophthalmol. 2001; 49:255–259. PMID: 12930118.
16. Mishima K, Tomidokoro A, Suramethakul P, et al. Iridotrabecular contact observed using anterior segment three-dimensional OCT in eyes with a shallow peripheral anterior chamber. Invest Ophthalmol Vis Sci. 2013; 54:4628–4635. PMID: 23761081.

17. Kerman BM, Christensen RE, Foos RY. Angle-closure glaucoma: a clinicopathologic correlation. Am J Ophthalmol. 1973; 76:887–895. PMID: 4759849.

18. Sacca SC, Gandolfi S, Bagnis A, et al. The outflow pathway: a tissue with morphological and functional unity. J Cell Physiol. 2016; 231:1876–1893. PMID: 26754581.
19. Meyer MA, Savitt ML, Kopitas E. The effect of phacoemulsification on aqueous outf low facility. Ophthalmology. 1997; 104:1221–1227. PMID: 9261307.
20. Lam DS, Leung DY, Tham CC, et al. Randomized trial of early phacoemulsification versus peripheral iridotomy to prevent intraocular pressure rise after acute primary angle closure. Ophthalmology. 2008; 115:1134–1140. PMID: 18164064.

Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download