Abstract
Purpose
In the present study, we investigated the treatment efficacy and clinical outcomes of botulinum neurotoxin-A (BoNT-A) administered for longer than 5 years to patients with essential blepharospasm.
Methods
We retrospectively reviewed 19 patients (male : female = 8 : 11) diagnosed with essential blepharospasm between March 2006 and July 2016 who underwent BoNT-A injections for over 5 years and were followed. Efficacy of 297 injections of Botox (n = 162), Meditoxin (n = 75), Hugel-tox (n = 40), or Dysport (n = 20) was based on the symptom improvement score at the final injection (−1, worse; 0, same; 1, better). Injection dose (botox unit), duration of efficacy (months), and adverse events were also investigated.
Results
Based on product type, significant differences in patient age (59.3 ± 9.8 years), disease period (5.0 ± 5.4 years), number of botulinum neurotoxin injections before visiting our clinic (1.6 ± 2.6), and follow-up period (7.2 ± 1.6 years) were not observed. Treatment efficacy score and injection dose of repetitive injections were 0.1 ± 0.5 and 39.1 ± 4.0 units, respectively, and did not show significant differences with repeated injections. Duration of response was 5.9 ± 5.4 months, but this significantly decreased as the injections were repeated (p < 0.01). Among the 297 injections, adverse events occurred 12 times (4.0%) with no severe sequelae.
Conclusions
In this study, we showed that repetitive, long-term BoNT-A injections are considered a stable and effective treatment for essential blepharospasm in terms of consistent injection dose and maintenance of treatment efficacy. However, the duration of long-term efficacy could be decreased in patients injected repetitively.
Essential blepharospasm is a syndrome in which overexcitement of the orbicularis oculi muscle and its adjacent muscles causes continuous excessive closure of the eyelids [1]. Following Food and Drug Administration approval, injection of botulinum neurotoxin-A (BoNT-A) into the orbicularis oculi muscle has been considered the primary treatment for essential blepharospasm. The safety of BoNT-A was demonstrated because it functions without making permanent changes in the terminal nerve tissues of targeted muscles [2]. BoNT-A brands currently used with proven efficacy in the domestic market are Botox (Allergan Inc., Irvine, CA, USA), Dysport (Ipsen Ltd, Berkshire, UK), Hugel-tox (Hugel, Seoul, Korea), Xeomin (Merz Pharmaceuticals, Frankfurt am Main, Germany), Prosigne (Lanzhou Biological Products, Lanzhou, China), and Meditoxin (Medy-Tox, Seoul, Korea).
Although there are many reports on the short-term efficacy of various BoNT-A types for treatment of essential blepharospasm, studies on long-term injection efficacy and adverse events among patients are limited. BoNT-A injection is the preferred treatment for essential blepharospasm and is widely used for various indications including the firming of periocular wrinkles. Studies on long-term efficacy of BoNT-A in blepharospasm, the representative disease for injection, are necessary. Therefore, in the present study, the treatment efficacy and clinical outcomes of BoNT-A injected for more than 5 years were investigated.
The present study initially included 268 patients diagnosed with essential blepharospasm who received BoNT-A injections at our hospital from March 2006 to July 2016. Patients who received neurosurgery or periocular resection or took anticonvulsants for other neurological disorders were excluded. The medical records, including number of injections (297), of 19 patients who received long-term treatment and were followed at our hospital for more than 5 years were retrospectively analyzed. Patients who experienced no efficacy with BoNT-A injections at other hospitals before visiting our facility were included in the study.
After cleansing the injection sites with frozen disinfectant, 100 units of BoNT-A (Botox, Meditoxin, and Hugel-tox) were diluted with 2.5 mL of saline solution and subcutaneously injected with a 1-mL syringe. Dysport (500 units) was diluted with 4.2 mL of saline solution. One oculoplastic surgeon injected the solution into the orbicularis oculi muscle located at the orbital septum, both inside and on the outskirt of the eyelid where twitching occurs, and into the corrugator supercilii, using the typical injection method previously reported [3]. Cold compression was applied for 5 minutes before and after injection.
The brands used for injection were Botox (n = 162), Meditoxin (n = 75), Hugel-tox (n = 40), and Dysport (n = 20), and treatment efficacy was graded based on symptom improvement (−1, worse; 0, same; 1, better) at every patient visit by comparing the efficacy between visits. Injection dose was performed at a 1 : 3 ratio between Dysport and BoNT-A with Botox. Duration of response was calculated as the time until the next re-injection. Additionally, possible complications after BoNT-A injections were investigated.
Statistical analysis was performed with Windows IBM SPSS ver. 23 (IBM Corp., Armonk, NY, USA) using variance analysis, regression analysis, independent sample t-test, and chi-square test; a p-value of 0.01 or less was considered statistically significant.
Among the 19 observed patients, based on the number of BoNT-A types used, the average age of 11 patients (7 females and 4 males) who received 4 types of injections was 61.7 ± 8.9 years (range, 42 to 72 years), and their average disease period was 6.3 ± 6.3 years. The average number of BoNT-A injections received due to blepharospasm before visiting our hospital was 1.6 ± 2.3, and the average follow-up duration was 7.8 ± 1.6 years. The average age of 4 patients (2 females and 2 males) who received 3 types of BoNT-A was 51.3 ± 8.1 years (range, 40 to 58 years) and the average disease period was 4.0 ± 4.2 years. The patients received on average 0.8 ± 1.0 BoNT-A injections before visiting our hospital, and the average follow-up duration was 6.3 ± 1.4 years. The average age of 3 patients (1 female and 2 males) who received 2 types of BoNT-A was 58.7 ± 13.6 years (range, 43 to 67 years), and the average disease period was 2.0 ± 3.5 years. The patients received on average 3.0 ± 5.2 BoNT-A injections before visiting our hospital, and the average follow-up duration was 6.9 ± 1.7 years. One patient, a 67-year-old female, received 1 type of BoNT-A injection with a disease period of 4 years, did not receive any previous BoNT-A injections, and had a follow-up duration of 5.6 years. Based on the number of BoNT-A injection types, differences were not observed in age, gender, disease period, the number of injections received before initial visit, or follow-up duration (Table 1 and Fig. 1).
Nineteen patients received a total of 297 injections; Botox (n = 162), Meditoxin (n = 75), Hugel-tox (n = 40), and Dysport (n = 20). The average age of the patients who received Botox was 62.1 ± 7.9 years. Overall, 106 injections were given to females and 56 injections to males. The average disease period was 5.7 ± 5.3 years, and the average number of previously administered BoNT-A injections was 1.8 ± 2.7. The average follow-up duration was 7.9 ± 1.5 years, and the average dose was 39.53 ± 12.31 units. The average interval was 7.19 ± 8.93 months. The average age of the patients who received Meditoxin was 58.1 ± 10.8 years. Overall, females received 44 injections and males received 31 injections. The average disease period was 5.9 ± 5.7 years, and the average number of previously administered BoNT-A injections was 1.3 ± 2.2. The average follow-up duration was 7.3 ± 1.6 years, and the average dose was 39.06 ± 8.57 units. The average interval was 6.23 ± 3.64 months. The average age of the patients who received Hugel-tox was 61.4 ± 9.2 years; females received 24 injections and males received 16 injections. The average disease period was 5.0 ± 5.1 years, and the average number of previously administered BoNT-A injections was 2.2 ± 3.0. The average follow-up duration was 7.8 ± 1.6 years, a nd the average dose was 35.14 ± 9.28 units. The average interval was 4.30 ± 1.32 months. The average age of the patients who received Dysport was 60.9 ± 9.0 years; females received 13 injections and males received 7 injections. The average disease period was 6.6 ± 6.3 years, and the average number of previously administered BoNT-A injections was 1.7 ± 2.3. The average follow-up duration was 7.7 ± 1.5 years, and the average dose was 46.60 ± 10.95 units. The average interval was 4.97 ± 2.05 months. Based on the BoNT-A type, differences were not observed in age, gender, disease period, the number of injections administered before visiting our hospital, follow-up duration, injection dose, or injection interval (Table 2).
During the follow-up period, there were a minimum of 9 to a maximum of 27 injections administered per each patient (Fig. 2). Treatment efficacy grading based on symptom improvement at the next visit after injection was 0.1 ± 0.5 point on average, and this score did not show significant differences with repeated injections (Table 3 and Fig. 3).
The average injection dose was 39.1 ± 4.0 units, which did not show significant difference based on the number of injections (Table 3 and Fig. 4). The average time until the next BoNT-A re-injection was 5.9 ± 5.4 months, which significantly decreased as injections were repeated (p < 0.01) (Table 3 and Fig. 4). Based on regression analysis, the equation Y=−0.12X + 7.106 was computed and after the 21st injection, and the injection time interval significantly decreased to 4.6 months (p < 0.01).
Among 297 injections, 12 (4.0%) adverse events were observed, and the patients experienced lagophthalmos, asymmetric mouth corner, blepharoptosis, and photophobia, all of which improved without any severe sequelae (Table 4).
BoNT-A improves blepharospasm symptoms when injected into the orbicularis oculi muscles and has been used as a n effective treatment for blepharospasm for over 20 years [4]. When BoNT-A, which is composed of a heavy chain joined via disulfide bonds to a light chain, is administered into the adjacent part of the targeted muscle, the heavy chain combines with a receptor at the cholinergic nerve terminal and enters into the muscle cell as a neurotoxin. BoNT-A prevents muscle contraction by inhibiting acetylcholine from being released at the presynaptic nerve ending; however, this is only temporary, and the contraction reoccurs when nerves sprout [5]. Clinical studies on changes in clinical efficacy when BoNT-A is used long-term and repetitively are lacking.
In a previous research comparing efficacy among currently used BoNT-A products, Dysport showed a 3–4 : 1 conversion rate with Botox, and Xeomin showed a 1 : 1 conversion rate with Botox. Reportedly, clinical efficacy was not different among injection types [6789]. Currently, no set standard exists for the dosage amount per ounce including conversion rate between different BoNT-A products or dosage amount per muscle. However, in the present study, we used Dysport at a 3 : 1 conversion rate with Botox, a nd efficacy a nd a dverse e vents a ppeared similar. Other BoNT-A products showed similar efficacy and safety with a 1 : 1 conversion rate with Botox [10], which was in agreement with our results.
Previous studies on treatment efficacy and clinical outcomes when BoNT-A is injected continuously over a long period for the treatment of blepharospasm have not included analysis of continuous injections. Most of the studies focused on comparison between the initial and final injections, ignoring all other injections. Based on a previous study that included facial dystonia patients, Ababneh et al. [11] compared only initial and final injections after injecting a single type of BoNT-A into 32 blepharospasm or hemifacial spasm patients over 14.1 years, injection dose increased while adverse events decreased from 11.5% in the first year to 3.8% in the final year with no severe sequelae.
Recently, Ramirez-Castaneda and Jankovic [12] compared only initial and final injections after injecting 5 types of BoNT-A into 89 dystonia patients (including 34 blepharospasm patients) for 18.6 years on average; the injection dose, maximum response, and duration of total response significantly increased and time to efficacy decreased. Overall, adverse events were reported in 19% of cases, with no severe sequelae.
We aimed to inject the smallest dose possible for the patients to adequately experience the twitch relaxation effect; as a result, the injection amount did not change significantly after more than 5 years of long-term injections. However, after the 21st injection, the time to the next injection decreased significantly, and we concluded that the efficacy duration period of BoNT-A could decrease in cases with repetitive injections. This result showed some correlation with previous reports showing that the injection dose increased and should be considered when injecting BoNT-A to blepharospasm patients.
Czyz et al. [13] have studied the frequency of adverse events after injecting 2 types of BoNT-A into 37 blepharospasm, hemifacial spasm, or Meigs syndrome patients for 19.4 years on average, and the frequency of patient side-effect complaints decreased to 20% compared to the initial injection, with no severe sequelae. The rate of adverse events was 4.0% in this study, which is lower compared with previous studies, indicating that our injection method is comparatively safe.
In this study, we evaluated blepharospasm patients repetitively injected with BoNT-A for a minimum of 5 years for changes in effects and clinical outcomes. Therefore, unlike other previous studies that only compared results from the initial and last injections excluding changes in middle stages, the present study is meaningful because results from every injection were continuously compared.
Additionally, although patients who received orbicularis oculi muscle resection or facial nerve decompression to directly treat blepharospasm were excluded, the elderly and patients who received eyelid surgery such as blepharoplasty, eyelid laxity repair, or intraocular surgery such as cataract or glaucoma, which might have indirectly and slightly affected blepharospasm, were included.
A limitation in the present study was inclusion of patients who received 4 types of products, because using only 1 type of BoNT-A product can lead to more accurate results regarding treatment tolerability or bodily reactions. However, insisting on a single product in an actual clinical setting is difficult. Since there is no difference in clinical efficacy among many of the currently used BoNT-A products based on the existing research, we conducted this study without limiting the number of product types. Additional studies on prospective long-term analysis using a single product are needed in the future.
Figures and Tables
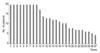 | Fig. 2The numbers of the patients according to the total numbers of botulinum neurotoxin-A injections. |
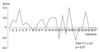 | Fig. 3The long-term result of repetitive botulinum neurotoxin-A injection in the blepharospasm patients. |
 | Fig. 4Injection dose (unit) and injection interval (mon) of repetitive botulinum neurotoxin-A injection in the blepharospasm patients. |
Table 1
Demographics and clinical characteristics of blepharospasm patients based on the number of botulinum neurotoxin-A types* used

Table 2
Demographics and clinical characteristics of blepharospasm patients based on botulinum neurotoxin-A type

References
1. Kenney C, Jankovic J. Botulinum toxin in the treatment of blepharospasm and hemifacial spasm. J Neural Transm (Vienna). 2008; 115:585–591.

2. Naumann M, Albanese A, Heinen F, et al. Safety and efficacy of botulinum toxin type A following long-term use. Eur J Neurol. 2006; 13:Suppl 4. 35–40.

3. Sung Y, Nam SM, Lew H. Clinical outcomes of individualized botulinum neurotoxin type A injection techniques in patients with essential blepharospasm. Korean J Ophthalmol. 2015; 29:115–120.

4. Scott AB, Kennedy RA, Stubbs HA. Botulinum A toxin injection as a treatment for blepharospasm. Arch Ophthalmol. 1985; 103:347–350.

6. Sampaio C, Ferreira JJ, Simoes F, et al. DYSBOT: a single-blind, randomized parallel study to determine whether any differences can be detected in the efficacy and tolerability of two formulations of botulinum toxin type A (Dysport a nd Botox) a ssuming a ratio o f 4:1. Mov Disord. 1997; 12:1013–1018.
7. Rieder CR, Schestatsky P, Socal MP, et al. A double-blind, randomized, crossover study of prosigne versus botox in patients with blepharospasm and hemifacial spasm. Clin Neuropharmacol. 2007; 30:39–42.

8. Pagan FL, Harrison A. A guide to dosing in the treatment of cervical dystonia and blepharospasm with Xeomin: a new botulinum neurotoxin A. Parkinsonism Relat Disord. 2012; 18:441–445.

9. Shin JH, Jeon C, Woo KI, Kim YD. Clinical comparability of dysport and botox in essential blepharospasm. J Korean Ophthalmol Soc. 2009; 50:331–335.

10. Yoon JS, Kim JC, Lee SY. Double-blind, randomized, comparative study of Meditoxin versus Botox in the treatment of essential blepharospasm. Korean J Ophthalmol. 2009; 23:137–141.

11. Ababneh OH, Cetinkaya A, Kulwin DR. Long-term efficacy and safety of botulinum toxin A injections to treat blepharospasm and hemifacial spasm. Clin Exp Ophthalmol. 2014; 42:254–261.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download