Abstract
Purpose
We describe our experience with the Permacol graft in anophthalmic socket reconstruction, and compare it to the autologous buccal mucosal graft, emphasizing the postoperative vascularization and contraction of each graft.
Methods
This was a retrospective comparative study. We measured the time necessary for the graft surface to be completely vascularized, as well as the fornix depth of the conjunctival sac in anophthalmic patients.
Results
Ten patients underwent Permacol graft reconstruction, with 44 undergoing buccal mucosal graft reconstruction. Seven eyelids (70%) in the Permacol group had a good outcome, with improvement in lower eyelid position and prosthesis retention. Nine out of 10 eyelids (90%) in this group showed complete vascularization of the graft at 2.6 ± 1.9 months postoperatively, while the grafted buccal mucosa was fully vascularized at 1.1 ± 0.3 months postoperatively (p < 0.01). Postoperative fornix depth in the Permacol group was 9.1 ± 2.2 mm, compared to 14.9 ± 4.5 mm in the buccal mucosal graft group (p < 0.01). Mean increases in fornix depth were 33.1% and 67.9% of the mean vertical length of the implanted graft.
Socket contracture sometimes occurs in anophthalmic patients, and this results not only in a poor cosmetic outcome, but also functional problems, such as ocular prosthesis displacement. To correct socket contracture, anterior lamellar repositioning or augmentation of the posterior lamella of the eyelid is usually required [12].
Current eyelid spacers include autogenous grafts, such as hard palate mucosal grafts. Allogeneic grafts include donor sclera or acellular human dermal graft (e.g., AlloDerm; LifeCell, The Woodlands, TX, USA), and synthetic implants include porous polyethylene. However, none of these substitutes are ideal as they are associated with disadvantages like donor site morbidity, risk of disease transmission, and implant migration and extrusion.
Recently, a novel material derived from porcine acellular dermis (Enduragen; Tissue Science Laboratories, Aldershot, UK) was described for use as a spacer graft in the eyelid. The study demonstrated a favorable outcome when the implant was used in the upper lid, lower lid, or as lateral canthal reinforcement [3]. This porcine acellular dermis graft has also been marketed by Covidien (Dublin, Ireland) under a different name, Permacol. In this study, we would like to introduce our experience with the Permacol graft by comparing it to the autologous buccal mucosal graft. In particular, we would like to highlight and compare the postoperative vascularization and contraction that takes place with each graft over a 6-month period.
This was a retrospective comparative study of patients who underwent socket reconstructive surgery using the Permacol graft or autologous buccal mucosal graft as a spacer at Severance Hospital, Yonsei University from January 2011 to December 2012, by a single surgeon (JSY). Only patients with an anophthalmic socket, with at least 6 months of follow-up after surgery were included. Medical records and clinical photographs of these patients were reviewed. Demographic data, follow-up duration, duration of prosthesis wear, cause of anophthalmos, risk factors for socket contracture, combined or further surgery, and complications of surgery were tabulated. Operation records, including size of implanted graft, were also obtained.
A good outcome was defined as an improvement in lower lid sagging with no prosthesis prolapse. A poor outcome was defined as no improvement in lower lid sagging that required further surgery with another type of spacer material, the inability to fit a prosthesis, or prosthesis prolapse. The time necessary for the graft surface to be completely vascularized was assessed to evaluate the degree of fibrovascular integration of the graft. These were thoroughly assessed using a slit lamp microscope. The fornix depth of the conjunctival sac was directly measured using a ruler, after removal of the artificial eye. The calculated postoperative fornix length was used to estimate immediate postoperative fornix depth. It was computed as the sum of preoperative fornix depth and vertical length of the implanted graft.
The institutional review board of Yonsei University College of Medicine, Seoul, Korea, approved this study. The study adhered to the tenets of the Declaration of Helsinki, and written informed consent was obtained from all participants.
A transconjunctival incision was made along the inferior border of the lower eyelid tarsus using monopolar cautery. Blunt dissection towards the orbital rim was performed without damaging the orbital septum. Upon reaching the orbital rim, an incision of the periosteum was made with a #15 blade, and an elevator was used to dissect the subperiosteal pocket to reach the infraorbital foramen.
In the Permacol graft group, the implant was trimmed to the size of the defect. In the autologous buccal mucosal graft group, an ellipse of full-thickness mucosal graft was harvested from the inner aspect of the cheek. Care was taken to avoid Stenson's duct adjacent to the upper second molar. Bosmin solution (epinephrine 1 mg/mL; Daiichi Sankyo, Tokyo, Japan) with 10% dilution application and electrocauterization were used at the donor site for hemostasis, and the defect was packed with gel-form. The harvested graft was trimmed to size, and submucosal tissue was removed with scissors.
The edges of the free graft were sutured to the surrounding conjunctiva with interrupted 6-0 absorbable braided polyglactin sutures (Vicryl; Ethicon, Livingston, UK). A temporary traction suture was maintained for 3 days postoperatively in all patients. Two double 5-0 non-absorbable monofilaments polyamide 6 (Nylon; WooRhi Medical, Namyangju, Korea) were used to anchor a silicone band bolster to deepen the inferior fornix. Each arm was passed through the silicone band and grafted mucosa, the periosteum of the inferior orbital rim, and out through the lower eyelid. Retinal sponge bolsters were used for the skin. A conformer was inserted in the socket for at least 4 weeks postoperatively. The surgical technique is presented in Fig. 1A-1D.
Patient characteristics, including age, duration of prosthesis wear, calculated fornix length, preoperative fornix depth, vertical length of implanted graft, and postoperative outcomes, including time to vascularization and postoperative fornix depth were compared between the groups using the Mann-Whitney test. Preoperative and postoperative fornix depth in each group was compared using Wilcoxon signed-rank test. All statistical tests were two-sided with an α-level of 0.05, and were performed using SPSS Statistics ver. 20.0. (IBM Co., Armonk, NY, USA).
Ten patients underwent Permacol graft reconstruction. Patient demographics and clinical characteristics are described in Table 1. The mean age was 38.2 years (range, 7 to 69 years), with an equal number of males and females (Table 2). They were followed for 12.5 ± 4.9 months with an average duration of prosthesis wear of 24.4 years (range, 1 to 55 years). The causes of anophthalmia were trauma (n = 3, 30%), congenital microphthalmia (n = 3, 30%), previous malignancy (n = 2, 20%), enucleation of eyeball after loss of vision by fever (n = 1, 10%), and unknown (n = 1, 10%).
Seven patients (70%) were classified as having a good outcome due to improvement in the lower eyelid position with prosthesis retention (Fig. 2A-2D). Three patients (patients 1, 8, and, 10) had a poor outcome. Two of these patients (patients 1 and 10) had persistent socket inflammation with recurrent socket contracture, requiring a second fornix deepening procedure with oral mucosal grafting. One patient (patient 8) was unable to retain an ocular prosthesis at the end of the follow-up period, despite a deepened fornix.
Nine out of 10 eyelids showed complete vascularization of the graft within 2 to 2.5 months of surgery (Fig. 3A). In one case (patient 5), the Permacol graft in the remaining eyelid did not vascularize well even at 8 months postoperatively (Fig. 3B). However, the unvascularized remnant islands were completely excised without complications.
The mean preoperative fornix depth was 4.4 ± 2.5 mm. The postoperative fornix depth at 1 month postoperatively was 11.4 ± 2.6 mm (p < 0.01), though this became shallower at 9.1 ± 2.2 mm (p < 0.01) at postoperative 6 months.
Patient ages ranged from 10 to 79 years with a mean age of 45.9 years, 17 patients (38.6%) were male (Table 2). The mean follow-up period was 8.4 months after the reconstructive surgery. Patients used an ocular prosthesis for an average of 15.6 years. The causes of anophthalmos included trauma (24 patients), tumor (five patients), congenital anomalies such as microphthalmos or anophthalmos (eight patients), and other disorders causing phthisis (e.g., retinal detachment, glaucoma, and uveitis) (seven patients).
In this group, 14 had severe socket contraction prior to the surgery; two had history of radiotherapy before enucleation for tumors; nine had previous complicated implant surgeries (e.g., exposure, infection, or small implant); three had a previous peg insertion; and two had prolonged socket inflammation for more than 1 month after socket reconstruction surgery.
The grafted buccal mucosa was fully vascularized approximately 1 month after surgery (mean ± standard deviation, 1.1 ± 0.3 months). At preoperative and postoperative 6 months, fornix depths were 5.8 ± 2.4 and 14.9 ± 4.5 mm, respectively (p < 0.01).
Age, sex, and duration of prosthesis wear were not significantly different between the two groups (Table 2). The Permacol graft took significantly longer (2.6 ± 1.9 months) to vascularize, compared to the buccal mucosal graft (1.1 ± 0.3 months) (p < 0.01). The preoperative fornix depths were not statistically different, as was the vertical length of the implanted graft (p = 0.274 and p = 0.573, respectively). The calculated postoperative fornix length did not differ between the groups (p = 0.274). In contrast, the fornix depth in the Permacol group was significantly shorter 9.1 ± 2.2 mm at the end of the follow-up period, compared to the fornix depth in the buccal mucosal graft group 14.9 ± 4.5 mm (p < 0.01) (Fig. 4). Mean increases in fornix depth after surgery were 4.4 mm and 9.1 mm, and they corresponded with 33.1% and 67.9% of the mean vertical lengths of the implanted graft, respectively.
The Permacol surgical implant is a bioengineered, porcine-derived dermal collagen implant, from which cells are removed in a gentle process to reduce immunogenicity. The resulting acellular collagen matrix is then cross-linked for enhanced durability, which allows the formation of new collagen in the matrix. Permacol was first described in the literature for use in reconstructive hand surgery [4]. More recently, it has been used in the repair of abdominal wall defects [5], orbital fractures [6], and eyelid reconstructions [37].
McCord et al. [3] have described a porcine acellular dermal collagen matrix manufactured by Tissue Science Laboratories as spacer material for the reconstruction of the upper and lower eyelid, as well as for lateral canthal suspension surgery.
To our knowledge, this is the first study to examine the clinical characteristics, as well as the vascularization and contracture of the Permacol graft, in detail in context of implantation in the lower eyelid as spacer material in anophthalmic socket reconstruction. We chose to compare it with buccal mucosal tissue, which is the preferred lower eyelid spacer material in our practice for anophthalmic socket reconstruction.
One major advantage of Permacol is that it is readily available to the surgeon and does not inflict any donor site morbidity. Since there is no need to harvest any donor tissue and the tissue does not require hydration prior to use, surgical time is reduced. The buccal mucosal graft, on the other hand, results in donor site morbidity, patient discomfort and prolongs surgical time as a result of donor harvesting [89]. One possible disadvantage of Permacol is that, like other xenografts (e.g., Tarsys), it may carry with it a potential risk of allergic reaction and inflammation [1011], though this was not encountered in our study population. It has also been reported to elicit a chronic granulomatous reaction, similar to a foreign body reaction, when implanted in the orbit for orbital floor fracture repair [6]. The buccal mucosal graft, on the other hand, is an autogenous source, and hence avoids the risk of tissue rejection and disease transmission.
In our clinical trial, the Permacol graft provided a good clinical outcome in seven out of 10 patients. The poor outcome in two patients was attributed to persistent socket inflammation with recurrent socket contracture. The third patient had a poor outcome despite a deepened fornix due to multiple factors, such as like facial nerve palsy, traumatic blow in fracture of the orbit, and absent orbital implant, which made prosthesis fitting impossible. The Permacol graft was completely vascularized in nine out of 10 patients within 2.5 months. This is not surprising, as the vascularization process of the implanted porcine acellular dermis graft has been demonstrated to take place as early as the third postoperative day in rat models [12]. However, vascularization of the graft was poor in one patient (patient 5). It is possible that vascularization is dependent on the surface area in contact with the host conjunctival tissue, as demonstrated in human acellular dermis grafts [13]. Any local host tissue factors that interfere with this interaction could affect the vascularization of the graft (e.g., an undetected hematoma, graft slippage, infection, or an irradiated socket with poor vascular supply). Systemic conditions like poorly controlled diabetes with poor wound healing response may also affect the vascularization of the graft. The duration to vascularization in the Permacol group was significantly longer than in the buccal mucosal group. Animal histologic studies have shown that small vessels and inflammatory cells are present up to 5 weeks after implantation [14], suggesting that the host remodeling response is ongoing at this stage. This delay in vascularization could be attributed to delayed host cell recognition of the acellular xenograft, in contrast with an autogenous buccal mucosal graft.
In this study, direct measurement of the immediate postoperative fornix depth could not be completed due to postoperative discomfort. Instead, we used the calculated postoperative fornix length to estimate it. The calculated postoperative fornix length is computed as the sum of the preoperative fornix depth and the vertical length of the grafted material, but is not the actual postoperative fornix depth because the angulation of the fornix was formed on graft material, duplicating the graft length in the fornix; in other words, the actual fornix depth may be shorter than the calculated fornix length. However, the calculated fornix length was not statistically different between the two groups, and a fornix depth difference at 6 months after surgery implies clinical significance.
The authors feel that Permacol is not as effective as buccal mucosal graft as a fornix-deepening spacer material. There was an improvement in fornix depth in the Permacol group, but the improvement was significantly less than that afforded by the buccal mucosa group. This is most likely due to fibrosis and contraction of the Permacol graft over time. Significant graft shrinkage of the porcine acellular dermal matrix was noted in the in vivo rat model [14]. Graft shrinkage of the acellular human dermis graft has previously been described [151617], with resorption appearing to be a primary disadvantage. The rate and occurrence of resorption is unpredictable, and has been suggested to be due to inadequate exposure to vascular tissue and dehydration [13]. It seems likely that the porcine acellular dermis graft undergoes a similar process of fibrosis, contraction, and resorption. As such, the authors recommend oversizing the Permacol graft intraoperatively, in order to account for graft shrinkage over time.
The limitations of this study are its retrospective design and limited duration of follow-up. It also has a small sample size, and reflects only a single surgeon's experience.
In summary, the Permacol graft can be a useful spacer graft material in patients with anophthalmic sockets. Careful selection of patients with no risk factors for recurrent socket contracture and poor vascularization is important. Meticulous surgical technique will optimize the surgical outcome, and oversizing the graft intraoperatively will help to compensate for postoperative graft shrinkage.
Figures and Tables
Fig. 1
The surgical procedure using the Permacol implant in anophthalmic socket reconstruction. (A) Intraoperative appearance of Permacol implant. The implant edge is sutured to the conjunctival edge. (B) External appearance of anophthalmic socket with fornix deepening sutures and retinal bolsters in place. (C) Appearance of graft at 1 week postoperatively. (D) Appearance of graft at 1 month postoperatively with superficial vascularization.
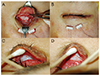
Fig. 2
Representative patients in preoperative (left) and postoperative (right) photographs, who underwent reconstructive surgery with the Permacol graft. Photographs of adult (A,B) and pediatric (C,D) anophthalmic socket patients.
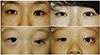
Fig. 3
Conjunctivalization of the Permacol graft. (A) Good outcomes showing complete vascularization of the Permacol graft. (B) Poor vascularization of the Permacol graft at 8 months postoperatively, showing unvascularized islands.

Fig. 4
Comparison of preoperative (preop) and postoperative (postop) fornix depth in anophthalmic socket patients receiving Permacol and undergoing buccal mucosal graft reconstructive surgery. *p < 0.05, both groups.

References
1. Hashikawa K, Terashi H, Tahara S. Therapeutic strategy for the triad of acquired anophthalmic orbit. Plast Reconstr Surg. 2007; 119:2182–2188.
2. Yoshizawa M, Feinberg SE, Marcelo CL, Elner VM. Ex vivo produced human conjunctiva and oral mucosa equivalents grown in a serum-free culture system. J Oral Maxillofac Surg. 2004; 62:980–988.
3. McCord C, Nahai FR, Codner MA, et al. Use of porcine acellular dermal matrix (Enduragen) grafts in eyelids: a review of 69 patients and 129 eyelids. Plast Reconstr Surg. 2008; 122:1206–1213.
4. Belcher HJ, Zic R. Adverse effect of porcine collagen interposition after trapeziectomy: a comparative study. J Hand Surg Br. 2001; 26:159–164.
5. Liyanage SH, Purohit GS, Frye JN, Giordano P. Anterior abdominal wall reconstruction with a Permacol implant. J Plast Reconstr Aesthet Surg. 2006; 59:553–555.
6. Cheung D, Brown L, Sampath R. Localized inferior orbital fibrosis associated with porcine dermal collagen xenograft orbital floor implant. Ophthal Plast Reconstr Surg. 2004; 20:257–259.
7. Peter NM, Kumar B. Permacol in eyelid reconstruction: a novel use. Orbit. 2013; 32:57–59.
8. Klein M, Menneking H, Bier J. Reconstruction of the contracted ocular socket with free full-thickness mucosa graft. Int J Oral Maxillofac Surg. 2000; 29:96–98.
9. Molgat YM, Hurwitz JJ, Webb MC. Buccal mucous membrane-fat graft in the management of the contracted socket. Ophthal Plast Reconstr Surg. 1993; 9:267–272.
10. Liao SL, Wei YH. Correction of lower lid retraction using tarSys bioengineered grafts for graves ophthalmopathy. Am J Ophthalmol. 2013; 156:387–392.e1.
11. Kim HJ, Grossniklaus HE, Wojno TH. A cyst-like foreign body reaction to porcine decellularized membrane (TarSys). Ophthal Plast Reconstr Surg. 2014; 30:e100–e102.
12. Xie WG, Tan H, Zhao CL, Wang H. The histological changes and the revascularization process in the grafted dermal substitutes. Zhonghua Shao Shang Za Zhi. 2005; 21:37–39.
13. Rubin PA, Fay AM, Remulla HD, Maus M. Ophthalmic plastic applications of acellular dermal allografts. Ophthalmology. 1999; 106:2091–2097.
14. Liu Z, Tang R, Zhou Z, et al. Comparison of two porcine-derived materials for repairing abdominal wall defects in rats. PLoS One. 2011; 6:e20520.
15. Sullivan SA, Dailey RA. Graft contraction: a comparison of acellular dermis versus hard palate mucosa in lower eyelid surgery. Ophthal Plast Reconstr Surg. 2003; 19:14–24.
16. Owens KW, Yukna RA. Collagen membrane resorption in dogs: a comparative study. Implant Dent. 2001; 10:49–58.
17. Gryskiewicz JM, Rohrich RJ, Reagan BJ, Schwartz BM. The use of Alloderm for the correction of nasal contour deformities. Plast Reconstr Surg. 2001; 107:571.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download