Abstract
Purpose
To report the outcome of scleral buckling using a non-contact wide-angle viewing system with a 25-gauge chandelier endoilluminator.
Methods
Retrospective analyses of medical records were performed for 17 eyes of 16 patients with primary rhegmatogenous retinal detachment (RRD) without proliferative vitreoretinopathy who had undergone conventional scleral buckling with cryoretinopexy using the combination of a non-contact wide-angle viewing system and chandelier endoillumination.
Results
The patients were eight males and five females with a mean age of 26.8 ± 10.2 (range, 11 to 47) years. The mean follow-up period was 7.3 ± 3.1 months. Baseline best-corrected visual acuity was 0.23 ± 0.28 logarithm of the minimum angle of resolution units. Best-corrected visual acuity at the final visit showed improvement (0.20 ± 0.25 logarithm of the minimum angle of resolution units), but the improvement was not statistically significant (p = 0.722). As a surgery-related complication, there was vitreous loss at the end of surgery in one eye. As a postoperative complication, increased intraocular pressure (four cases) and herpes simplex epithelial keratitis (one case) were controlled postoperatively with eye drops. One case of persistent RRD after primary surgery needed additional vitrectomy, and the retina was postoperatively attached.
Conclusions
Scleral buckling with chandelier illumination as a surgical technique for RRD has the advantages of relieving the surgeon's neck pain from prolonged use of the indirect ophthalmoscope and sharing the surgical procedure with another surgical team member. In addition, fine retinal breaks that are hard to identify using an indirect ophthalmoscope can be easily found under the microscope by direct endoillumination.
Rhegmatogenous retinal detachment (RRD) is a sight-threatening disease that can cause permanent vision loss unless surgically repaired. Options for the treatment of RRD include pneumatic retinopexy, pars plana vitrectomy (PPV), and scleral buckling [1]. Cardinal principles for the surgery are identification of all retinal breaks, treatment of breaks with laser or cryotherapy, and release of associated vitreoretinal traction [2]. Although the standard treatment of RRD has been scleral buckling, a shift in treatment choice toward PPV has occurred in recent years with the improved instrumentation and safety of PPV. Young patients with inferior retinal breaks might still prefer to undergo scleral buckling rather than PPV. Traditional scleral buckling is a good surgical option because it is an extraocular procedure with low costs and decreased postoperative complications, including unexpected vitreous loss and new retinal breaks or vitreous hemorrhage [3]. In scleral buckling, the retina is visualized using an indirect ophthalmoscope, which has the disadvantages of an inverted, small fundus image and the inability to share fundus information with an assistant [24567]. In addition, prolonged surgery using the indirect ophthalmoscope can cause neck pain and fatigue for the surgeon.
In this study, we report the results of a modified buckling procedure using a non-contact wide-angle viewing system with a 25-gauge chandelier endoilluminator.
This study was a retrospective analysis of the medical records of 17 eyes of 16 patients with RRD who had undergone scleral buckling surgery using a non-contact wide-angle viewing system (Resight; Carl Zeiss Meditec AG, Jena, Germany) with a 25-gauge chandelier endoilluminator (Chandelier Lighting System; Alcon Laboratories, Fort Worth, TX, USA). Scleral buckling was performed by a single surgeon, and all patients underwent at least 3 months of postoperative follow-up. The following variables were analyzed: sex, age, preoperative visual acuity, final visual acuity, follow-up period, preoperative and postoperative intraocular pressure (IOP), first and final anatomical success rate, lens status, number and size of retinal breaks, location of retinal breaks, number of quadrants involved, presence or absence of macular involvement, buckling material, postoperative complications, and operative time. For operation time, the first five cases were excluded to account for the procedural learning curve. Operation times were compared with times for conventional scleral buckling procedure cases performed by the same surgeon in the past. The study was approved by our institutional review board (2016-1063) and followed the principles of the Declaration of Helsinki. Also written informed consents were obtained.
After the administration of general anesthesia, conjunctival peritomy was performed to expose the sclera. Four extraocular rectus muscles were isolated, and traction sutures with 4-0 black silk were inserted underneath each muscle. One sclerotomy with a 25-gauge trocar cannula was made 3.5 mm behind the limbus on the opposite side of the retinal break. The 25-gauge chandelier illuminator was inserted into the vitreous cavity (Fig. 1A). A non-contact wide-angle viewing system provided a clear view of the fundus under the illuminator inserted into the vitreous cavity (Fig. 1B). The retinal break was identified (Fig. 2), and cryoretinopexy of the retinal break was performed by scleral compression using a cryoprobe (Fig. 3). The buckling procedure was then performed using a silicone sponge for encircling or a tire for segmental buckling. After confirmation of the buckle position, the 25-gauge sclerotomy site was closed using a 6-0 vicryl suture and completely covered by conjunctiva at the end of surgery.
Table 1 shows preoperative demographic data for all patients. In total, 17 eyes from 16 patients (10 males and 6 females) were included. The mean patient age was 26.8 ± 10.2 (range, 11 to 47) years, and the mean postoperative follow-up period was 7.3 ± 3.1 (range, 3 to 12) months. All patients were phakic, and their mean refractive error was -3.9 ± 3.9 diopters (spherical equivalent). The mean number of retinal breaks was 1.5 ± 0.8. Retinal breaks were localized at the superior retina in seven eyes, the inferior retina in eight eyes, and the temporal retina in two eyes. The size of the breaks was 2.0 ± 3.0 disc size with the exception of one case, which had a giant tear. Five eyes had RRD involving one quadrant, nine had RRD involving two quadrants, and three had more extensive RRD involving three or four quadrants. We found new breaks during surgery in four eyes. In three cases, an unidentified tiny break was found during surgery, and one case had retinal dialysis. Mean preoperative best-corrected visual acuity was 0.23 ± 0.28 logarithm of the minimum angle of resolution units. Mean postoperative best-corrected visual acuity at 1 month and at the final visit were 0.23 ± 0.23 and 0.20 ± 0.25 logarithm of the minimum angle of resolution units, respectively. These changes were not statistically significant (p = 0.656 and 0.722, respectively; Wilcoxon signed rank test). Six patients had undergone segmental buckling and 11 patients had undergone encircling buckling.
The mean operative time for all patients was 76.8 ± 16.1 minutes, which excluded the first five patients as part of the learning curve. The operation time of the 17 cases with the conventional scleral buckling procedure performed by the same surgeon was 93 ± 11.7 minutes, which was significantly different from the operation time of this study (p = 0.037, Wilcoxon signed rank test). The anatomical success rate for a single surgery was 94.1% (16 / 17). This rate is higher than the known primary success rate (approximately 80%) of conventional scleral buckling [2], and is consistent with previous reports (Table 2) [2589]. PPV was performed on one eye because of a failure of buckling. The final anatomical success rate was 100% (17 / 17). One eye had an intraoperative complication, and five eyes had postoperative complications. The intraoperative complication was loss of vitreous, which was treated by the removal of the leaked vitreous using Westcott scissors. Postoperative complications were increased IOP (≥25 mmHg) in four eyes and herpes simplex epithelial keratitis in one eye. They were treated with IOP-lowering agents and a systemic antiviral agent, respectively. A comparison of these results with other previously reported studies is shown in Table 2 [2589].
In this study, a modified scleral buckling procedure improved visualization during surgery and relieved surgeon fatigue resulting from prolonged use of the indirect ophthalmoscope. With advancements in surgical instruments, including a small-gauge intraocular illumination system, conventional surgical technique can be improved to help meet surgical need and reduce discomfort during surgery. One of the main limitations of the indirect ophthalmoscope is that it offers a limited surgical view. Consequently, the surgeon's view cannot be shared with assistants. Use of the wide-angle viewing system with a microscope overcame this limitation. Based on recent reports on the high rate of occupational musculoskeletal disorders in vitreoretinal surgeons, comfortable methods for performing surgery are important [1011]. Exact visualization and magnification of retinal breaks during surgery under a surgical microscope is an additional advantage of this technique. Based on a previous report comparing conventional and modified scleral buckling procedures, operation time of the modified scleral buckling procedure was significantly shorter than that of the conventional scleral buckling procedure [2]. This result can be confirmed in operation time analysis of the two surgical methods performed by the same surgeon in this study. This might be due to the reasons described above, although additional time might be required to insert the 25-gauge trocar and place the chandelier. Other recent reports also suggest the usefulness of the wide-angle viewing system for performing scleral buckling [4567].
Possible disadvantages of this surgical method include light toxicity, lens damage, vitreous loss during surgery, and infective endophthalmitis [12]. In our small case series, we did not observe chandelier-related serious complications, such as vitreous hemorrhage, endophthalmitis, hypotony, or lens touch. We observed one case of vitreous loss through the sclerotomy site, which was resolved with manual cutting and meticulous suturing. Four patients had increased IOP, which could have been prevented with paracentesis of the anterior chamber during surgery.
The limitations of our study are its retrospective nature and the small number of subjects, which make it difficult to compare the advantages of conventional and modified scleral buckling procedures. Large, randomized, prospective, comparative studies are needed to compare modified scleral buckling, conventional scleral buckling, and primary PPV procedures.
Scleral buckling using a non-contact wide-angle viewing system with a chandelier endoilluminator is effective and safe for the reattachment of RRD, particularly if retinal breaks are difficult to preoperatively detect. The surgical outcome of this procedure was comparable to that of the conventional scleral buckling procedure. Moreover, the procedure reduces surgeon discomfort and fatigue and enables assistant residents and fellows to better learn the scleral buckling procedure.
Figures and Tables
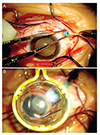 | Fig. 1(A) A 25-gauge chandelier was inserted into the inferotemporal quadrant after isolation of the rectus muscle. The location of the chandelier was determined based on the location of the retinal break, 90° or 180° from the retinal break. (B) A non-contact wide-angle viewing system provides a clear view of the fundus. |
References
1. D'Amico DJ. Clinical practice: primary retinal detachment. N Engl J Med. 2008; 359:2346–2354.
2. Narayanan R, Tyagi M, Hussein A, et al. Scleral buckling with wide-angled endoillumination as a surgical educational tool. Retina. 2016; 36:830–833.
3. Adelman RA, Parnes AJ, Ducournau D. European Vitreo-Retinal Society (EVRS) Retinal Detachment Study Group. Strategy for the management of uncomplicated retinal detachments: the European vitreo-retinal society retinal detachment study report 1. Ophthalmology. 2013; 120:1804–1808.
4. Ohji M, Tano Y. Vitreoretinal surgery with slit-lamp illumination combined with a wide-angle-viewing contact lens. Am J Ophthalmol. 2004; 137:955–956.
5. Aras C, Ucar D, Koytak A, Yetik H. Scleral buckling with a non-contact wide-angle viewing system. Ophthalmologica. 2012; 227:107–110.
6. Nam KY, Kim WJ, Jo YJ, Kim JY. Scleral buckling technique using a 25-gauge chandelier endoilluminator. Retina. 2013; 33:880–882.
7. Kita M, Fujii Y, Kawagoe N, Hama S. Scleral buckling with a noncontact wide-angle viewing system in the management of retinal detachment with undetected retinal break: a case report. Clin Ophthalmol. 2013; 7:587–589.
8. Imai H, Tagami M, Azumi A. Scleral buckling for primary rhegmatogenous retinal detachment using noncontact wide-angle viewing system with a cannula-based 25 G chandelier endoilluminator. Clin Ophthalmol. 2015; 9:2103–2107.
9. Nagpal M, Bhardwaj S, Mehrotra N. Scleral buckling for rhegmatogenous retinal detachment using vitrectomy-based visualization systems and chandelier illumination. Asia Pac J Ophthalmol (Phila). 2013; 2:165–168.
10. Mehta S, Hubbard GB 3rd. Avoiding neck strain in vitreoretinal surgery: an ergonomic approach to indirect ophthalmoscopy and laser photocoagulation. Retina. 2013; 33:439–441.
11. Shaw C, Bourkiza R, Wickham L, et al. Mechanical exposure of ophthalmic surgeons: a quantitative ergonomic evaluation of indirect ophthalmoscopy and slit-lamp biomicroscopy. Can J Ophthalmol. 2017; 52:302–307.
12. Yokoyama T, Kanbayashi K, Yamaguchi T. Scleral buckling procedure with chandelier illumination for pediatric rhegmatogenous retinal detachment. Clin Ophthalmol. 2015; 9:169–173.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download