Abstract
Purpose
To investigate the prevalence and risk factors for an epiretinal membrane (ERM) in Korean population.
Methods
Using the database of the Korea National Health and Nutrition Examination Survey from 2008 through 2012, 14,772 participants 40 years of age or older with gradable fundus photographs were included. The presence of ERM was determined by using fundus photographs. The prevalence of ERM was estimated and possible risk factors including systemic factors, nutritional status, and blood tests were analyzed via multiple logistic regression analyses.
Results
The prevalence of ERM was 2.9% (95% confidence interval [CI], 2.6% to 3.3%). On multiple logistic regression analysis, the prevalence of ERM was affected by age. The odds ratios (ORs) against the forties were 2.70, 5.48, and 5.69 in the fifties, sixties, and seventies, respectively. ERM was also significantly affected by cataract surgery (OR, 2.82; 95% CI, 2.08 to 3.81) and by the increase in intake of 100-mg calcium (OR, 1.05; 95% CI, 1.00 to 1.11). ERM had negative associations with red blood cell count (OR, 0.66; 95% CI, 0.45 to 0.95).
Epiretinal membrane (ERM) is the most common type of fibrocellular proliferation which is found at the vitreoretinal interface. With aging population, the number of patients undergoing surgical intervention for ERM is increasing. Previous epidemiologic studies addressed the prevalence of ERM, and the findings from those studies indicate a range from 1.0% to 28.9% [123456789101112131415]. However, as far as the present authors know, there has been no study representing a nationwide population in Asia. Difference in prevalence of ERM by country and ethnicity is widely known and is thought to be caused by genetic or lifestyle factors [16]. ERM contains cells whose functionality is reflected through calcium dynamics upon acetylcholine and mechanical stimulation [17]. Kokavec et al. [18] have reported that the concentration of glucose in the vitreous was correlated with that in the serum. Animal studies have reported that dietary protein imbalance and cholesterol-enriched diet can be toxic to retinal tissue or result in changes to retinal structures [1920]. Some epidemiological studies have indicated that ERM is associated with retinal arteriolar narrowing [611], which, in turn, could be influenced by sodium intake [21]. Based on these results, it is suspected that some systemic and nutrition factors influence the occurrence of ERM. However, there has been no study investigating risk factors for ERM, including socioeconomic conditions and nutrition status. The Korea National Health and Nutrition Examination Survey (KNHANES) is a nationwide cross-sectional survey which represents entire Korean population of approximately 50 million. The purpose of the current study was to investigate the prevalence and risk factors of ERM in Korea utilizing the data from the KNHANES.
The KNHANES is an ongoing, population-based, cross-sectional survey in South Korea conducted by the Korea Centers for Disease Control and Prevention and the Korean Ministry of Health and Welfare. The present study analyzed the data of the 2008 through 2012 KNHANES. This survey represented the civilian, non-institutionalized Korean population by using rolling sampling design with a complex, stratified, multistage, probability-cluster survey. Not a simple random sample, but the quoted design is used widely in health surveys to sample a fraction of large finite population while accounting for its size and characteristics. In this design, sampling is always multistage, using strata (separate sampling from population subgroups), cluster (considering the possibility of groups of observations), and weight (considering oversampling or undersampling) [22]. In KNHANES, both the 1-year data surveys and the integrated data of the 2008 through 2012 surveys represent the entire population of Korea. In this study, data from a total of 20,419 eligible subjects 40 years of age or older during the 5-year study period was analyzed. The institutional review board of the Samsung Medical Center (no. 2016-02-091) approved the present study, which was conducted in accordance with the Declaration of Helsinki.
The KNHANES consisted of three components: the health interview survey, the nutrition survey, and the health examination. Health interview data were composed of basic demographics, socioeconomic status, and standardized questionnaires regarding health-related problems. For the nutrition survey, trained interviewers asked participants about dietary behavior and food frequency questionnaires. From this data, the amount of intake of each nutrient was estimated according to the composition table of the Rural Developmental Administration. Nutrient variables analyzed included calories, carbohydrates, proteins, fat, fiber, ash, calcium, phosphorus, Fe, sodium, potassium, vitamin A, beta-carotene, retinol, thiamin (B1), riboflavin (B2), niacin, and vitamin C. Health examination surveys consisted of basic body measurements, laboratory tests for blood and urine, chest X-ray, a bone density test, and physical examinations such as otorhinolaryngologic and ophthalmic examinations. Ophthalmic examination included corrected visual acuity test along with refraction, intraocular pressure measurement, slit lamp examination, visual fields test, and fundus photography. Fundus photographs were obtained with a nonmydriatic fundus camera (TRC-NW6S; Topcon, Tokyo, Japan). Patients were defined as having ERM if a cellophane macular reflex or premacular fibrosis was observed in the fundus photograph. A cellophane macular reflex was defined as a patchy, irregular, or increased light ref lection on the inner retinal surface. Premacular fibrosis was defined as the presence of a grayish or opaque appearance with superficial retinal folds on the inner retinal surface. Each fundus photograph was graded by two experienced retinal specialists (SJP and JSS). The present study used the data from all of these surveys; basic demographics, socioeconomic status, medical histories, anthropometric investigations, nutritional status, blood tests, ophthalmic surveys, and ophthalmic examinations including fundus photography.
The variables analyzed in this study were defined and categorized as follows: the first category among the categories of each variable defined below was selected as a reference in logistic regression analysis (LRA). Participants were divided into 4groups according to their ages: 40 to 49 years, 50 to 59 years, 60 to 69 years, and 70 years of age or older. Education status was divided into three groups. House income status was divided into three groups. Residence was categorized as an urban or rural area based on the address of the participants. Smoking status was defined as a never smoker, a former smoker, or a current smoker. Drinking status was defined as a non-drinker or drinker. Comorbidity status was based on the presence or absence of any comorbid condition. Red blood cells (RBCs) were measured by a XE-2100D (Sysmex, Kobe, Japan). Other blood tests obtained included measurement of the blood urea nitrogen, creatinine, vitamin D, alkaline phosphatase, and parathyroid hormone.
Comparative analysis was conducted of participants included and excluded for this study and the prevalence of ERM was estimated. Simple LRAs and the chi-square test were conducted to investigate the associations between ERM prevalence and a set of variables. Then, the LRAs adjusted for age group and gender were performed. Covariates that had a p-value of less than 0.200 in each LRA adjusted for age group and gender were chosen for multiple LRAs. The data were analyzed with PASW Statistics ver. 18 (SPSS Inc., Chicago, IL, USA) using proc survey procedures, which can analyze the presented data properly using the variable of strata, cluster, and weight. This study used the KNHANES sample weight adjusted for oversampling and nonresponse in the Korean population from 2008 to 2012 [23]. General linear modeling and chi-square tests were conducted for the comparison of demographic characteristics according to the presence of ERM. A p-value less than 0.050 was considered statistically significant.
Among all participants of the KNHANES from 2008 through 2012, 20,419 subjects were 40 years of age or older and underwent ophthalmologic examinations. A total of 5,647 subjects did not have a gradable fundus photograph due to reasons including cataracts and lack of cooperation. The demographic characteristics of participants according to presence of an ERM are provided in Table 1. Of the 14,772 participants 40 years of age or older, an ERM was observed in 507 (Fig. 1). The estimated prevalence of ERM, adjusted by demographic structure in Korea, was 2.9% (95% confidence interval [CI], 2.6% to 3.3%) among the subjects aged more than 40 years. The estimated prevalence in the population aged more than 50 and 60 years was 4.2% (95% CI, 3.7% to 4.8%) and 6.0% (95% CI, 5.3% to 6.9%), respectively. Seventy-three subjects were found to have an ERM in both eyes (14.3%).
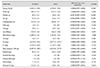
The distribution of visual acuity in the subjects with an ERM is demonstrated in Fig. 2. If both eyes were involved w ith ERM, the eye w ith worse vision was taken into account. As a whole, 65.7% (330 / 502) of the eyes had a decreased visual acuity ranging from 20 / 25 to 20 / 40. The age distribution among subjects with ERM was as follows: 12.5% (95% CI, 8.4% to 18.2%) of subjects in their forties; 24.0% (95% CI, 19.4% to 29.1%) in their fifties; 32.9% (95% CI, 28.2% to 38.0%) in their sixties; and 30.6% (95% CI, 26.0% to 35.7%) in their seventies. For subjects in their forties compared to the older age groups, the odds ratios (ORs) adjusted for age and gender were as follows: 2.28 for subjects in their fifties (p < 0.001), 6.62 in their sixties (p < 0.001), and 7.48 in their seventies (p < 0.001). The gender distribution of ERM was 37.5% in men and 62.5% in women. The age-adjusted OR in women was 1.39 (95% CI, 1.14 to 1.68; p = 0.001) (Table 1). The prevalence of ERM according to comorbidities is provided in Table 2. The proportion of current smokers was 29.3% (95% CI, 28.3% to 30.3%) in participants without ERM and 17.6% (95% CI, 13.8% to 22.2%) in those with ERM. The a ge a nd sex a djusted OR of being a c urrent smoker compared with nonsmokers was 0.64 (95% CI, 0.45 to 0.93; p = 0.018). The proportion of participants with a history of cataract surgery was 25.5% (137 / 507) in subjects with ERM and 6.3% (1,217 / 14,265) in those without ERM. The adjusted OR of cataract surgery was 2.85 (95% CI, 2.17 to 3.74; p < 0.001) (Table 2). The results of the age and sex adjusted OR for the intake of nutrients are provided in Table 3.
With lower intake of energy, water, protein, fat and carbohydrates was, there was higher the risk of ERM. Blood urea nitrogen and alkaline phosphatase levels had a significant OR in blood tests (Table 4). Multiple LRAs were performed to investigate the complex risk of ERM according to the variables with a p-value less than 0.200 after LRA (Table 5). For subjects in their forties compared to the older age groups, the prevalence of ERM had the following association by OR (95% CI): an OR of 2.70 (1.47 to 4.96) for patients in their fifties; an OR of 5.48 (3.02 to 9.94) for patients in their sixties; and an OR of 5.69 (3.06 to 10.56) for patients in their seventies. ERM was also significantly affected by a history of cataract surgery (OR, 2.82; 95% CI, 2.08 to 3.81) and the increase in intake of 100 mg of calcium (OR, 1.05; 95% CI, 1.00 to 1.11). The prevalence of ERM in women was more strongly associated with the intake of calcium compared to men, men having an OR of 1.02 (p = 0.593) a nd women having a n OR of 1.08 (p = 0.010). RBC count was also associated with the prevalence of ERM. If the RBC count was 1 million/µL higher, the risk of ERM was 0.66 times lower (p = 0.027). However, this result differed by the gender (men, OR 0.51 [p = 0.006]; women, OR 0.80 [p = 0.343]) (Table 5 and Fig. 3).
The prevalence of ERM in Beaver Dam Eye Study and Blue Mountains Eye Study was 11.8% and 7.0%, respectively [115]. In Japan, the Hisayama Study reported a prevalence of 4.0%. In Shanghai Study of China and the Beijing Eye Study reported the prevalence of ERM as 1.02% and 2.2%, respectively [312]. The present study provided detailed data on the prevalence and risk factors of ERM based on a larger sample representing nationwide population in Korea [7]. The estimated prevalence of ERM was 2.9% in the Korean population 40 years of age or older. The result is similar to the results found in studies of the population of China and Japan. As previous studies indicated, the prevalence of ERM in East Asia was apparently lower than that in western countries. However, a higher prevalence of ERM in Asian ethnicities has been reported in some multi-ethnic studies [610]. Thus, whether the large variability in the prevalence of ERM was affected by the ethnicity or environment remains unclear.
As for the risk factors for ERM, some reports claimed that female gender was associated with an increased risk of ERM [3614], while other reports claim that they could not find a gender-specific difference [58]. Analysis of refractive errors also showed mixed results in previous studies [6781114]. In the univariate analysis of the current study, both female gender and myopic refractive errors were related to an increased risk of ERM. However, multiple LRA indicated that both factors had no significant association with ERM. In this regard, it is worth noting that only a few of the previously described epidemiologic studies of ERM prevalence adopted multiple LRA for their risk analysis. There have been m ixed results regarding the association between diabetes and ERM [191215]. Hypercholesterolemia [31013] and a higher level of education [712] have also been reported as independent risk factors for ERM. These associations were not corroborated by the current study.
Increased age was associated with the prevalence of ERM, as it was confirmed in most of the previous studies. This may be due to the increase in posterior vitreous detachment (PVD) with aging. As in previous studies [1,241524], an association between cataract surgery and ERM prevalence was confirmed. Jahn et al. [24] reported that the prevalence of ERM increased from 14.8% to 25.3% six months after extracapsular cataract surgery. They explained that this was associated with PVD induced by cataract surgery and the resulting mechanical traction on the vitreo-retinal interface. Although the correlation of extracapsular cataract surgery with the prevalence of ERM was identified in previous studies, it remains to be answered whether modern cataract surgery involving smaller incisions and phacoemulsification techniques would also be related to an increased prevalence of ERM. The present study shows that modern cataract surgery can also be a risk factor for ERM.
According to the results of multiple LRA, increased intake of calcium was associated with an increased risk of ERM. Tajima et al. [25] reported that the synthesis of collagen decreased in cell cultures under hypercalcemic conditions. Reduced synthesis of collagen induces vitreous liquefaction and this may result in PVD. In addition, calcium itself may lead to changes in adhesion molecules at the vitreo-retinal interface, which may also result in PVD and subsequent ERM. Unrelated to PVD, a high intake of calcium may facilitate migration or proliferation of cellular components involved in ERM formation [26]. However, these are mere hypotheses and further studies are needed. Nevertheless, the present study showed that a high intake of calcium can be an independent risk factor for ERM. This may have important clinical implications, since calcium may act as a new therapeutic target for ERM prevention in the future.
RBC count was also associated with ERM. When RBC count decreased, the risk of ERM increased. Although it is hard to explain the relationship between anemia and ERM in men, a common mechanism found in the development of ERM in subjects with sickle cell anemia may work [2728]. Although further investigation is required to reveal any definite associations, the current study indicates that restricting calcium intake in women, and anemia correction in men may help reduce the incidence of ERM.
This study has several limitations. First, the occurrence of PVD could not be investigated and correlated with ERM prevalence, because grading was done only with fundus photographs. The prevalence of ERM was 2.9% in this study, which might be underestimated by using only fundus photography not optical coherence tomography. It was possible to miss tiny ERM in some cases. Moreover, since this study is a cross-sectional study, it has inherent limitations in explaining the causal relationship of pathogenesis. Second, both idiopathic and secondary ERM were included without discrimination. Third, although a high intake of calcium or a low RBC count were independently related to the prevalence of ERM, there might be other confounding factors that could not be assessed or adjusted for this study. Nevertheless, this study has its own strength as a population-based study representing the demographic structure of one nation. Also, the number of participants in this study is roughly fifteen thousand, which is large enough to conduct multiple LRAs for risk factor analysis. Moreover, as far as the authors are aware, this is the first study assessing risk factors for ERM with regard to socioeconomic conditions and nutrient intake.
In conclusion, the nation-wide prevalence of ERM was 2.9% in the Korean population aged 40 years or older. Risk f actors for ERM were aging, a history of cataract surgery, a high intake of calcium and a low RBC count.
Figures and Tables
 | Fig. 1Flow chart of subjects excluded and included for analysis in the current study. KHNANES = Korea National Health and Nutrition Examination Survey; ERM = epiretinal membrane. |
 | Fig. 3Forest plot in the multiple logistic regression model for risk factors of epiretinal membrane. |
Table 1
Demographic characteristics of participants according to the presence of ERM, and the result of age and sex adjusted univariate analysis
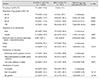
Table 2
Mean value or frequency of clinical features in the group without ERM and with ERM, and the result of age and sex adjusted univariate analysis

Table 3
The difference of nutrient intake between no ERM group and ERM group, and the result of age and sex adjusted univariate analysis
References
1. Mitchell P, Smith W, Chey T, et al. Prevalence and associations of epiretinal membranes: the Blue Mountains Eye Study, Australia. Ophthalmology. 1997; 104:1033–1040.
2. Fraser-Bell S, Guzowski M, Rochtchina E, et al. Five-year cumulative incidence and progression of epiretinal membranes: the Blue Mountains Eye Study. Ophthalmology. 2003; 110:34–40.
3. Miyazaki M, Nakamura H, Kubo M, et al. Prevalence and risk factors for epiretinal membranes in a Japanese population: the Hisayama Study. Graefes Arch Clin Exp Ophthalmol. 2003; 241:642–646.
4. Fraser-Bell S, Ying-Lai M, Klein R, et al. Prevalence and associations of epiretinal membranes in latinos: the Los Angeles Latino Eye Study. Invest Ophthalmol Vis Sci. 2004; 45:1732–1736.
5. McCarty DJ, Mukesh BN, Chikani V, et al. Prevalence and associations of epiretinal membranes in the visual impairment project. Am J Ophthalmol. 2005; 140:288–294.
6. Kawasaki R, Wang JJ, Mitchell P, et al. Racial difference in the prevalence of epiretinal membrane between Caucasians and Asians. Br J Ophthalmol. 2008; 92:1320–1324.
7. You Q, Xu L, Jonas JB. Prevalence and associations of epiretinal membranes in adult Chinese: the Beijing eye study. Eye (Lond). 2008; 22:874–879.
8. Duan XR, Liang YB, Friedman DS, et al. Prevalence and associations of epiretinal membranes in a rural Chinese adult population: the Handan Eye Study. Invest Ophthalmol Vis Sci. 2009; 50:2018–2023.
9. Kawasaki R, Wang JJ, Sato H, et al. Prevalence and associations of epiretinal membranes in an adult Japanese population: the Funagata study. Eye (Lond). 2009; 23:1045–1051.
10. Ng CH, Cheung N, Wang JJ, et al. Prevalence and risk factors for epiretinal membranes in a multi-ethnic United States population. Ophthalmology. 2011; 118:694–699.
11. Koh V, Cheung CY, Wong WL, et al. Prevalence and risk factors of epiretinal membrane in Asian Indians. Invest Ophthalmol Vis Sci. 2012; 53:1018–1022.
12. Zhu XF, Peng JJ, Zou HD, et al. Prevalence and risk factors of idiopathic epiretinal membranes in Beixinjing blocks, Shanghai, China. PLoS One. 2012; 7:e51445.
13. Aung KZ, Makeyeva G, Adams MK, et al. The prevalence and risk factors of epiretinal membranes: the Melbourne Collaborative Cohort Study. Retina. 2013; 33:1026–1034.
14. Ye H, Zhang Q, Liu X, et al. Prevalence and associations of epiretinal membrane in an elderly urban Chinese population in China: the Jiangning Eye Study. Br J Ophthalmol. 2015; 99:1594–1597.
15. Klein R, Klein BE, Wang Q, Moss SE. The epidemiology of epiretinal membranes. Trans Am Ophthalmol Soc. 1994; 92:403–425.
16. Bu SC, Kuijer R, Li XR, et al. Idiopathic epiretinal membrane. Retina. 2014; 34:2317–2335.
17. Andjelic S, Lumi X, Yan X, et al. Characterization of ex vivo cultured neuronal- and glial-like cells from human idiopathic epiretinal membranes. BMC Ophthalmol. 2014; 14:165.
18. Kokavec J, Min SH, Tan MH, et al. Biochemical analysis of the living human vit reous. Clin Exp Ophthalmol. 2016; 44:597–609.
19. Bonavolonta O, Ferrante P, Terracciano L, Vecchione R. Further researches about retinal damages and dietary protein imbalance in growing rats. Int J Vitam Nutr Res. 1991; 61:251–257.
20. Trivino A, Ramirez AI, Salazar JJ, et al. A cholesterol-enriched diet induces ultrastructural changes in retinal and macroglial rabbit cells. Exp Eye Res. 2006; 83:357–366.
21. Raff U, Harazny JM, Titze SI, et al. Salt intake determines retinal arteriolar structure in treatment resistant hypertension independent of blood pressure. Atherosclerosis. 2012; 222:235–240.
22. Oyeyemi GM, Adewara AA, Adeyemi RA. Complex survey data analysis: a comparison of SAS, SPSS and STATA. Asian J Math Stat. 2010; 3:33–39.
23. Kim Y, Park S, Kim NS, Lee BK. Inappropriate survey design analysis of the Korean National Health and Nutrition Examination Survey may produce biased results. J Prev Med Public Health. 2013; 46:96–104.
24. Jahn CE, Minich V, Moldaschel S, et al. Epiretinal membranes after extracapsular cataract surgery(1). J Cataract Refract Surg. 2001; 27:753–760.
25. Tajima T, Iijima K, Watanabe T, Yamaguchi H. The influence of calcium ions on the synthesis of collagen and glycosaminoglycans in human diploid cells in culture. Exp Pathol. 1981; 19:219–225.
26. Smith-Thomas L, Haycock JW, Metcalfe R, et al. Involvement of calcium in retinal pigment epithelial cell proliferation and pigmentation. Curr Eye Res. 1998; 17:813–822.
27. Carney MD, Jampol LM. Epiretinal membranes in sickle cell retinopathy. Arch Ophthalmol. 1987; 105:214–217.
28. Rajagopal R, Apte RS. Full-thickness macular hole in a patient with proliferative sickle cell retinopathy. Retina. 2010; 30:838–839.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download