Abstract
Purpose
To evaluate the association between degree of retinal abnormalities and uncorrected visual acuity (UCVA) in idiopathic epiretinal membrane (ERM) patients with a small amount of refractive error.
Methods
We retrospectively reviewed 49 eyes (37 patients) of idiopathic ERM patients. We investigated the association between visual acuity and macular status (central macular thickness [CMT], outer retinal integrity score, and inner retinal irregularity index) that was assessed by optical coherence tomography using multiple linear regression analysis. We defined visual acuity difference (VAD) as the difference between UCVA and best-corrected visual acuity (BCVA). We divided patients into two groups according to VAD size and compared clinical characteristics between the two groups. We also investigated factors associated with VAD using multiple linear regression analysis.
Results
BCVA showed significant association with CMT and outer retinal integrity score, while UCVA showed significant association with CMT and inner retinal irregularity index. Patients with a large VAD showed a similar level of BCVA compared to the small VAD group (logarithm of the minimum angle of resolution [logMAR], large VAD group 0.11 ± 0.11 vs. small VAD group 0.13 ± 0.12, p = 0.585). However, UCVA was worse (logMAR, large VAD group 0.44 ± 0.14 vs. small VAD group 0.18 ± 0.14, p < 0.001) and inner retinal irregularity was higher (large VAD group 1.06 ± 0.04 vs. small VAD group 1.04 ± 0.03, p < 0.001) in patients with a large VAD. On multiple linear regression analysis, the absolute value of spherical equivalent (standardized coefficient β 0.521, p < 0.001) and inner retinal irregularity index (standardized coefficient β 0.448, p < 0.001) were significantly associated with VAD.
Conclusions
UCVA was associated with inner retinal irregularity in idiopathic ERM patients with a mild degree of refractive error. Inner retinal irregularity was also associated with degree of VAD, suggesting that the effect of refractive error correction is greater in patients with more distorted retina.
The general process of visual perception is that light passing through the eye media including cornea, lens, and vitreous is transmitted to the photoreceptors of the retina, and image information is integrated in the brain [1]. Visual acuity refers to the ability to distinguish the details of the object and shape at a given distance [2]. Visual acuity is primarily influenced by eye condition, such as refractive errors and presence or absence of cataract or retinal disorders. Refractive errors such as myopia and astigmatism prevent images from accurately forming on the retina, which reduces vision [345].
Uncorrected visual acuity (UCVA) is defined as visual acuity measured without correcting refractive errors. Best-corrected visual acuity (BCVA) is examined after correcting refractive errors. The degree of difference between UCVA and BCVA depends on the amount of refractive error [6]. In our clinical experience, we have found that patients with minor refractive error showed good UCVA if they had good eye status including clear lens and intact retinal state. In contrast, patients with retinal abnormalities typically showed more decreased UCVA even with mild refractive error. In most clinical studies, visual function has been measured by BCVA after correcting refractive errors, and BCVA showed good correlation with retinal status [789101112]. Few studies have focused on UCVA, and there is little study on the relationship between UCVA and retinal state. We hypothesized that retinal status is related with UCVA as well as BCVA in mild refractive error cases, and the degree of difference between UCVA and BCVA will be greater in cases with retinal abnormalities. We therefore evaluated the association between degree of retinal abnormalities and UCVA in idiopathic epiretinal membrane (ERM) patients with small refractive errors.
This study was conducted according to the tenets of the Declaration of Helsinki. This was a retrospective, single-center study and was approved by the institutional review board of Kangdong Sacred Heart Hospital (2016-11-004). We reviewed the medical records of idiopathic ERM patients who visited our clinic from April 2014 to June 2016. Idiopathic ERM was diagnosed clinically by fundus examination using 90-diopter slit-lamp examination or indirect ophthalmoscopy and optical coherence tomography (OCT). The inclusion criterion was idiopathic ERM patients who had small refractive error (absolute spherical equivalent [SE] less than 2 diopters and astigmatism less than 1.5 diopters). Exclusion criteria were as follows: 1) patients with preexisting ocular pathologies such as age-related macular degeneration, diabetic retinopathy, retinal vein occlusion, or advanced glaucoma; 2) patients with ocular trauma history; 3) patients with a history of intraocular surgery other than uncomplicated cataract surgery; 4) patients with more than N2 or C2 grade cataract by the Lens Opacities Classification System III classification; or 5) preexisting corneal pathologies such as severe keratitis or corneal opacity.
All patients underwent comprehensive ophthalmologic examination including UCVA, BCVA (Snellen visual acuity chart), intraocular pressure, refractive errors (represented as SE and astigmatism), slit lamp examination, and OCT (Spectralis; Heidelberg Engineering, Heidelberg, Germany). Refractive errors were measured with an auto kerato-refractometer (KR-8900; Topcon, Tokyo, Japan). The UCVA, BCVA, and refractive error values measured at the time of first identification of idiopathic ERM in the clinic were used for the analysis. All patients underwent OCT with horizontal and vertical scans across the fovea center and 30° × 20° volume scan. Central macular thickness (CMT) was defined as average retinal thickness within the central 1mm zone of the Early Treatment Diabetic Retinopathy Study (ETDRS) grid, and macular volume was defined as the central 6-mm zone retinal volume of the ETDRS grid. Average retinal nerve fiber layer (RNFL) thickness in the ETDRS central 1-mm area and RNFL volume in the ETDRS 6-mm area were also evaluated. These values were calculated automatically by built-in auto-segmentation software, and one observer (LJH) reviewed and manually adjusted segmentation errors. We evaluated the integrity of the outer retinal layer using horizontal scan images centered on the fovea. We evaluated the integrity of the external limiting membrane, ellipsoid zone, and interdigitation zone within a center 3-mm area. A score of 2 was assigned for clearly visible and continuous lines, 1 for partially disrupted and discontinuous lines, and 0 for severely disrupted and indistinguishable lines for each outer retinal hyperreflective line observed on OCT. We defined outer retinal integrity score as the sum of these three line scores, which ranged from 0 to 6 (Fig. 1). We also evaluated the degree of inner retinal irregularity by measuring the inner retinal irregularity index recently introduced by Cho et al. [7]. In brief, inner retinal irregularity index was defined as the ratio of the length of the inferior border of the inner plexiform layer to the length of the retinal pigment epithelium layer within a center 3-mm zone. Measurements were conducted using Image J (available in the public domain at http://rsb.info.nih.gov/ij/) with Neuron J, a semi-automated layer-tracing plug-in module for Image J.
All continuous variables are reported as mean ± standard deviation values. Visual acuities were converted to logarithm of the minimum angle of resolution (logMAR) scale for statistical analysis. The association between patient visual acuity and macular status was assessed by multiple linear regression analysis. The difference between UCVA and BCVA was defined as visual acuity difference (VAD), and we divided patients into two groups, small and large VAD groups, according to the VAD median value (0.1 logMAR). We compared clinical characteristics including CMT, RNFL thickness, outer retinal integrity, and inner retinal irregularity between the two groups using Student's t-test and chi-square or Fisher's exact test for continuous and categorical variables, respectively. We also performed multiple linear regression analysis to investigate clinical factors associated with VAD. Statistical analyses were performed using IBM SPSS ver. 23.0 (IBM Corp., Armonk, NY, USA). A p-value less than 0.05 was considered statistically significant.
During the study period, 83 eyes of 63 patients were diagnosed with idiopathic ERM. Ten eyes with age-related macular degeneration and 7 eyes with severe cataract were excluded. Seventeen eyes that showed refractive error exceeding the inclusion criteria were also excluded. In total, 49 eyes of 37 patients were included in this study. The demographic and clinical characteristics of the patients are summarized in Table 1. The mean age of the patients was 70.0 ± 10.1 years, mean BCVA was 0.12 ± 0.12 (logMAR, Snellen equivalent 0.8), and mean UCVA was 0.30 ± 0.19 (logMAR, Snellen equivalent 0.5). Mean VAD was 0.18 ± 0.18 (logMAR) with median value of 0.10. We investigated the association between visual acuity and retinal status represented by CMT, outer retinal integrity score, and inner retinal irregularity index. BCVA showed significant association with both CMT (standardized coefficient β 0.350, p = 0.035) and outer retinal integrity score (standardized coefficient β −0.292, p = 0.047), while there was no significant association between inner retinal irregularity index and BCVA (standardized coefficient β −0.049, p = 0.736) (Table 2). In contrast, UCVA showed significant association with CMT (standardized coefficient β 0.421, p = 0.005) and inner retinal irregularity index (standardized coefficient β 0.302, p = 0.024), but not with outer retinal integrity score (standardized coefficient β −0.079, p = 0.540) (Table 3).
We divided patients into two groups based on degree of VAD. There were no significant differences between the small and large VAD groups in terms of age, sex, or refractive error. Interestingly, the two groups showed similar BCVA, although UCVA was significantly better in the small VAD group. On retinal status assessment, the large VAD group showed significantly thicker CMT with larger macular volume and increased inner retinal irregularity index, while there were no significant differences in average RNFL thickness and outer retinal integrity score. We also investigated factors associated with VAD. On multiple linear regression analysis, we included the absolute value of SE and astigmatism in the model as potential factors related with VAD. We then added factors of age, sex, CMT, RNFL thickness, outer retinal integrity score, and inner retinal irregularity index into the model using a stepwise approach. The final model revealed that only absolute value of SE (standardized coefficient β 0.521, p < 0.001) and inner retinal irregularity index (standardized coefficient β 0.448, p < 0.001) were significantly associated with VAD (Table 4).
In this study, we evaluated the association between the degree of retinal abnormalities, as CMT, outer retinal integrity score, and inner retinal irregularity index, and UCVA in idiopathic ERM patients. Unlike BCVA, which showed significant correlation with CMT and outer retinal integrity score, UCVA had significant correlation with CMT and inner retinal irregularity index. Patients with a large VAD showed a similar level of BCVA compared to the small VAD group; however, the UCVA was worse and CMT was thicker. Inner retinal irregularity index was also increased in the large VAD group compared to the small VAD group. On multiple linear regression analysis, absolute value of SE and inner retinal irregularity index were significantly associated with VAD.
Among morphological changes of the retina in idiopathic ERM patients observed in OCT, outer retinal integrity seems to be related to potential visual acuity. This is consistent with previous studies showing that ellipsoid zone or interdigitation zone is related to BCVA [789101112]. UCVA was not associated with outer retinal integrity, but was significantly correlated with CMT and inner retinal irregularity index. Retinal distortion is caused by ERM, and these retinal changes are considered to lower UCVA. Patients included in this study had a mean outer retinal integrity score of 4.8 ± 1.3, and most patients had only limited damage to the outer retina. In these patients, refractive error correction might indicate significant improvement in visual acuity.
The process of image formation remains elusive but is thought to be a combination of multiple processes. The image analyzing process is divided into five phases: preprocess, detection, segmentation, registration, and interpretation [13]. Even if the image formed in the eye is unclear, the brain has a multiple spatial scale that functions simultaneously to interpret and understand the image [13141516]. We can hypothesize that, in cases with good retinal state, blurred images due to mild refractive error could be compensated by integrative visual process. On the other hand, in patients with severe macular distortion, the visual system is unable to compensate for inaccurate image information associated with refractive errors. In this study, the small and large VAD groups showed similar levels of refractive error and BCVA; however, the large VAD group had poor UCVA, thicker CMT, and increased inner retinal irregularity. Inner retinal irregularity index was an important factor in determining VAD along with SE in our study population.
Our findings suggest that refractive error correction, even in small amounts, is important in patients with retinal disorders. In general, most patients are unaware of the necessity of mild refractive corrections, and clinicians also tend to ignore small refractive errors [171819]. However, we should recognize that refractive error correction is essential and could achieve substantial visual acuity improvement in patients with retinal disorders even if the degree of refractive error is small.
Our study has several limitations. First, metamorphopsia and decreased vision are major complaints in ERM patients. However, we could not collect patient metamorphopsia data due to the retrospective study design. Mechanical distortion of the inner retina is known to be correlated with the degree of metamorphopsia [20]. Further study investigating the correlation between degree of metamorphopsia and UCVA or VAD might be needed.
Second, in this study, 29 eyes were pseudophakic, and their preoperative refractive error is mostly unknown. Myopic eyes are thought to better tolerate retinal defocus than emmetropic eyes with wider range of depth of focus [212223]. It is plausible that those who have long been adapted to their myopic state might exhibit better UCVA even though their current refractive error changed after cataract surgery. The original refractive state should also be considered in future analyses
Third, patients included in this study had mild refractive errors and relatively intact outer retinal integrity. Therefore, expansion of our findings to patients with severe refractive errors or severe retinal damage is limited. Further studies with a larger number of patients demonstrating various degrees of refractive error and retinal disorders are also needed.
In conclusion, in idiopathic ERM patients with a mild degree of refractive error, UCVA showed good correlation with the degree of inner retinal irregularity. Inner retinal irregularity index was also associated with the degree of VAD. These findings suggest that the effect of refractive error correction is greater in patients with more distorted retina due to ERM.
Figures and Tables
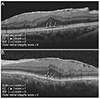 | Fig. 1Outer retinal integrity score. (A) Cases with intact outer retinal zones. External limiting membrane (ELM, arrows), ellipsoid zone (EZ, arrow heads), and interdigitation zone (IDZ, dashed arrows) are well visible and continuous. They were scored 2 for each zone and overall outer retinal integrity score becomes 6. (B) Cases with disrupted outer retinal zones. ELM (arrows) and EZ (arrow heads) are discontinuous and scored 1 for each zone. IDZ (dashed arrows) are not clearly differentiated and scored 0. The overall outer retinal integrity score becomes. |
Table 1
Demographic and clinical characteristics of included cases and comparison of the two groups divided by the degree of difference between best-corrected visual acuity and uncorrected visual acuity

Values are presented as mean ± standard deviation or number (%).
logMAR = logarithm of minimal angle resolution; SE = spherical equivalent; RNFL = retinal nerve fiber layer.
*The two groups were divided at 0.1 logMAR, which is the median value of the difference between best-corrected and uncorrected visual acuity; †Student's t-test and chi-square test or Fisher's exact test were used for continuous and categorical variables, respectively.
Table 2
Association between best-corrected visual acuity and macular status assessed by multiple linear regression analysis

Table 3
Association between uncorrected visual acuity and macular status assessed by multiple linear regression analysis

References
1. Westheimer G. Visual acuity: information theory, retinal image structure and resolution thresholds. Prog Retin Eye Res. 2009; 28:178–186.
2. Gilbert M. Definition of visual acuity. Br J Ophthalmol. 1953; 37:661–669.
3. Smith G. Relation between spherical refractive error and visual acuity. Optom Vis Sci. 1991; 68:591–598.
4. Waddell K. Spherical refraction for general eye workers. Community Eye Health. 2000; 13:6–7.
5. Eames TH. Correspondence between visual acuity, refractive error, and the speed of visual perception. Br J Ophthalmol. 1953; 37:312–313.
6. Lu YP, Xia WT, Chu RY, et al. Relationship between best corrected visual acuity and refraction parameters in myopia. Fa Yi Xue Za Zhi. 2011; 27:94–97.
7. Cho KH, Park SJ, Cho JH, et al. Inner-retinal irregularity index predicts postoperative visual prognosis in idiopathic epiretinal membrane. Am J Ophthalmol. 2016; 168:139–149.
8. Fang IM, Hsu CC, Chen LL. Correlation between visual acuity changes and optical coherence tomography morphological findings in idiopathic epiretinal membranes. Graefes Arch Clin Exp Ophthalmol. 2016; 254:437–444.
9. Aydın R, Karahan E, Kaya M, et al. Evaluation of inner segment/outer segment junctions in different types of epiretinal membranes. Arq Bras Oftalmol. 2016; 79:319–322.
10. Hosoda Y, Ooto S, Hangai M, et al. Foveal photoreceptor deformation as a significant predictor of postoperative visual outcome in idiopathic epiretinal membrane surgery. Invest Ophthalmol Vis Sci. 2015; 56:6387–6393.
11. Shimozono M, Oishi A, Hata M, et al. The significance of cone outer segment tips as a prognostic factor in epiretinal membrane surgery. Am J Ophthalmol. 2012; 153:698–704.
12. Itoh Y, Inoue M, Rii T, et al. Correlation between foveal cone outer segment tips line and visual recovery after epiretinal membrane surgery. Invest Ophthalmol Vis Sci. 2013; 54:7302–7308.
13. Ryan SJ, Schachat AP, Wilkinson CP, et al. Retina. 5th ed. Philadelphia: Elsevier/Saunders;2013. p. 156–160.
14. Wegener D, Galashan FO, Markowski DN, Kreiter AK. Selective visual attention ensures constancy of sensory representations: testing the influence of perceptual load and spatial competition. Vision Res. 2006; 46:3563–3574.
15. Xu H, Liu P, Dayan P, Qian N. Multi-level visual adaptation: dissociating curvature and facial-expression aftereffects produced by the same adapting stimuli. Vision Res. 2012; 72:42–53.
16. Marrugo AG, Sorel M, Sroubek F, Millan MS. Retinal image restoration by means of blind deconvolution. J Biomed Opt. 2011; 16:116016.
17. Marmamula S, Keeffe JE, Raman U, Rao GN. Populationbased cross-sectional study of barriers to utilisation of refraction services in South India: Rapid Assessment of Refractive Errors (RARE) Study. BMJ Open. 2011; 1:e000172.
18. Naidoo KS, Chinanayi FS, Ramson P, Mashige KP. Rapid assessment of refractive error in the eThekwini Municipality of KwaZulu-Natal, Durban, South Africa. Clin Exp Optom. 2016; 99:360–365.
19. Mashayo ER, Chan VF, Ramson P, et al. Prevalence of refractive error, presbyopia and spectacle coverage in Kahama District, Tanzania: a rapid assessment of refractive error. Clin Exp Optom. 2015; 98:58–64.
20. Ichikawa Y, Imamura Y, Ishida M. Metamorphopsia and tangential retinal displacement after epiretinal membrane surgery. Retina. 2017; 37:673–679.
21. Gwiazda J, Thorn F, Bauer J, Held R. Myopic children show insufficient accommodative response to blur. Invest Ophthalmol Vis Sci. 1993; 34:690–694.
22. Rosenfield M, Abraham-Cohen JA. Blur sensitivity in myopes. Optom Vis Sci. 1999; 76:303–307.
23. Wang B, Ciuffreda KJ. Depth-of-focus of the human eye: theory and clinical implications. Surv Ophthalmol. 2006; 51:75–85.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download