Abstract
Purpose
To investigate the peripapillary choroidal thickness (PCT) of polypoidal choroidal vasculopathy (PCV) and exudative age-related macular degeneration (AMD) and to evaluate their responses to anti-vascular endothelial growth factor (VEGF).
Methods
Thirty eyes with PCV and 25 eyes with exudative AMD who were treatment naïve were included in this study. PCT and subfoveal choroidal thickness were evaluated both before and after intravitreal anti-VEGF.
Results
The initial mean PCT of PCV (153.78 ± 56.23 µm) was thicker than that of exudative AMD (88.77 ± 23.11 µm, p < 0.001). Temporal, superior, nasal, and inferior PCTs of PCV were all thicker than those observedin exudative AMD (all p < 0.05). After anti-VEGF, the mean PCT of PCV was significantly reduced (134.17 ± 41.66 µm, p < 0.001), but the same was not true not in exudative AMD (86.87 ± 22.54 µm, p = 0.392). PCTshowed a similar tendency in all quadrants.
Polypoidal choroidal vasculopathy (PCV) was originally described as a retinal disorder characterized by abnormalities of the choroidal vasculature, including the inner branching vascular networks, terminating in polypoidal lesions that can be seen on indocyanine green angiography (ICGA) [1]. PCV has been considered a variant of exudative age-related macular degeneration (AMD) because of their phenotypic similarities [2], but PCV and exudative AMD have differences in ethnic prevalence, natural history, and treatment responses [345]. These differences suggest that PCV and exudative AMD are two distinct diseases, with PCV being a primary abnormality of the choroid [6].
It is increasingly being observed that PCV has a thick subfoveal choroid [789]. Recently, several studies have reported that the subfoveal choroid is thicker in eyes with PCV than in those with typical exudative AMD [789], and that the subfoveal choroid is thicker in eyes with PCV with choroidal vascular hyperpermeability, suggesting different pathological mechanisms in PCV and exudative AMD [10]. It has been proposed that pachychoroid neovasculopathy, including pachychoroid pigment epitheliopathy, central serous chorioretinopathy, and PCV, is caused by a pachychoroid-driven process involving choroidal congestion and choroidal hyperpermeability manifested by choroidal thickening and dilated choroidal vessels [1112].
However, current studies on the choroidal thickness (CT) of PCV have been limited to the choroid of the macular region. Therefore, it is unclear whether the increased CT in patients with PCV is a general characteristic of the choroid or a localized phenomenon limited to the macula. Because the choroid is supplied by various capillary arteries according to regional distribution, the CT outside the macula, especially that in the peripapillary region, might reflect a different PCV pathophysiology. We therefore hypothesized that PCV has a different peripapillary choroidal thickness (PCT) and anti-vascular endothelial growth factor (anti-VEGF) response than exudative AMD. In the following study, we retrospectively investigated PCT and its response to anti-VEGF in eyes with PCV and exudative AMD.
This retrospective, comparative series was approved by the institutional review board of Yonsei University, Seoul, South Korea. Informed consent was obtained from all patients, and all study protocols adhered to the tenets of the Declaration of Helsinki. All data were collected from the Department of Ophthalmology, Severance Hospital, and all participants underwent a comprehensive ophthalmic examination, including measurement of best-corrected visual acuity, a dilated fundus examination, fundus fluorescein angiography, and ICGA with spectral domain optical coherence tomography (Spectralis OCT, ver. 1.5.12.0; Heidelberg Engineering, Heidelberg, Germany).
We retrospectively reviewed the medical records of patients with PCV or exudative AMD from January 2012 to June 2014. Inclusion criteria were as follows: (1) treatment for naïve PCV or exudative AMD, (2) more than 6 months of follow-up with anti-VEGF treatment (three consecutive monthly injections followed by a pro re nata protocol), and (3) optical coherence tomography (OCT) scan images of the peripapillary and macula regions at the initial visit and at the 6-month follow-up visit. Exclusion criteria included the presence of refractive errors >±3.0 diopters, amblyopia, significant cataract, obscuration of choroidal images due to the existence of significant media opacity or thick subfoveal hemorrhage, central geographic atrophy, a history of ocular inflammation, a history of retinal detachment, previous vitrectomy, intraocular surgery (including cataract surgery) in the study eye within 1 year, a history of ocular trauma, and glaucoma in the study eye. Eyes that underwent photodynamic therapy were also excluded.
PCV was diagnosed primarily on the basis of ICGA findings, branching vascular networks, and terminating polypoidal lesion(s). The diagnosis of exudative AMD was based on a combination of fundus fluorescein angiography and ICGA according to hyperfluorescence with late leakage associated with pigment epithelial detachment in the macular region, serous retinal detachment, subretinal exudation, or hemorrhage [13].
The peripapillary area was defined as the area within 3.4 mm from the center of the optic disk. CT was set as the vertical distance from the hyperreflective line of Bruch's membrane to the innermost hyperref lective line of the sclerochoroidal interface. A 360°, 3.4-mm-diameter circular OCT scan used the standard protocol for retinal nerve fiber layer assessment. Each sector was measured 90° from the temporal area, clockwise in the right eye and counter clockwise in the left eye (Fig. 1A-1F, 2A-2L). Thus, the 0° area of both eyes indicated the temporal peripapillary area, and the 90°, 180°, and 270° areas corresponded to the superior, nasal, and inferior PCT, respectively. PCT was measured using a previously reported method [14]. Briefly, using the modification tool in the OCT image viewer program, the segmentation line indicating the retinal pigment epithelium was modified to the sclerochoroidal junction by the investigator. With this modification, the chorioretinal thickness between the internal limiting membrane and the sclerochoroidal junction was measured. The CT was calculated by subtracting the retinal thickness from the chorioretinal thickness at each quadrant (Fig. 1). Measurement of subfoveal CT on the line scan image was performed manually with a built-in caliper tool in the OCT viewer program. Subfoveal CT was measured at the center of the fovea. If the retinal pigment epithelium line was not clearly defined because of pigment, epithelial detachment, or other abnormalities, Bruch's membrane was used as the inner margin of the choroid. If a hyporeflective band representing a suprachoroidal layer was seen on OCT, it was not included in the CT [15]. In cases with poorly defined chorioscleral junctions, the outer choroidal margin was demarcated as the line connecting the outer margin of the large choroidal vessel layer. Peripapillary and subfoveal OCT images were obtained at the initial presentation and again 6 months after anti-VEGF treatment. All measurements were performed by two independent observers (SHK and JML) in a blinded fashion, and the mean of these observations was used for analyses.
The baseline characteristics between PCV and exudative AMD were compared using chi-square tests for categorical variables and independent t-tests for continuous variables. All comparisons between PCV and exudative AMD were adjusted by age using an analysis of covariance. A paired t-test was carried out to compare results before and after anti-VEGF treatment in each group. A one-way analysis of variance test was used to determine possible differences between the sectors of the PCT. The reproducibility of CT measurements between the observers was calculated via intraclass correlation coefficients. All statistical analyses were performed using SPSS ver. 20.0 for Windows (IBM Corp., Armonk, NY, USA). A p-value <0.05 was considered statistically significant.
Thirty eyes with PCV and 25 exudative eyes with AMD were included in this study. The mean age of the PCV patients was 6.63 years younger than that of the exudative AMD patients (69.17 ± 7.78 and 73.80 ± 8.06 years, respectively, p = 0.038). There was no difference in other baseline characteristics, such as history of diabetes mellitus or hypertension, sex, lens status, or refractive error, between the PCV and the exudative AMD patients (Table 1).
The initial treatment agents were bevacizumab and ranibizumab. Two cases in each PCV and exudative AMD groups switched agent during the 6-month follow-up period (Table 2).
The initial PCT of PCV was thicker than that seen in exudative AMD. All quadrants showed a similar tendency (all p < 0.001). The mean initial PCT of PCV was 1.73-fold thicker than that of exudative AMD, and the difference between PCV and exudative AMD was 65.01 µm ( p < 0.001). The initial PCT difference was largest in the superior quadrant and lowest in the inferior quadrant (77.11 µm and 49.03 µm, respectively). The initial inferior PCT of PCV was thinner than those in the temporal and superior quadrants (p = 0.018 and p = 0.015, respectively) but not thinner than that of the nasal quadrant (p = 0.09). The initial inferior PCT of exudative AMD was thinner than those of the superior and nasal quadrants (p = 0.014 and p = 0.012, respectively) but not thinner than that of the temporal quadrant (p = 0.105) (Fig. 3).
After anti-VEGF treatment, the mean PCT of the PCV decreased by 12.75%, from 154.78 ± 56.23 µm to 134.17 ± 41.66 µm (p < 0.001). The PCT of the PCV after treatment of all quadrants showed a similar tendency (Fig. 3). The PCT reduction in PCV was greatest in the inferior quadrant and lowest in the superior quadrant (18.94%, 22.67 µm and 10.23%, 17.27 µm, respectively). However, in exudative AMD, there was no statistically significant difference following anti-VEGF treatment. The mean PCT of exudative AMD after treatment decreased by 2.14% from 88.77 ± 23.11 µm to 86.87 ± 22.54 µm (p = 0.392). The PCT reduction in exudative AMD was greatest in the superior quadrant and lowest in the nasal quadrant, but no quadrant differences were signif icant ( p = 0.23 and p = 0.948, respectively) (Table 3). Although the PCT of PCV decreased more than that of exudative AMD after treatment, the mean PCT of PCV was still thicker (1.53-fold) than that of exudative AMD, and the difference between PCV and exudative AMD was 47.3 µm (p < 0.001). The inferior PCT was also thinner than those of the other quadrants in both PCV and exudative AMD after treatment (p = 0.03 and p < 0.001, respectively). There was no difference in response between the types of anti-VEGFs.
The initial subfoveal CT of PCV was 128.35 µm, which was 1.75-fold thicker than that of exudative AMD (p < 0.001). After anti-VEGF treatment, both the subfoveal CT of PCV and exudative AMD were reduced (p < 0.001 and p = 0.021, respectively). However, the percentage reduction was greater in PCV than in exudative AMD (59.56 µm, 19.85% and 15.56 µm, 9.06%, respectively). The difference also decreased; the mean subfoveal CT of PCV after treatment was still thicker (1.54-fold) than that of exudative AMD, and the difference between PCV and exudative AMD was 84.35 µm (Table 3). There was also no difference in response according to type of anti-VEGF.
In terms of interobserver reproducibility, the intraclass correlation coefficients showed agreement between the two observers in all locations both initially and after treatment (p < 0.001). These values were high and ranged from 0.903 to 0.953.
Our results showed that all quadrants of PCT in PCV were larger than those of exudative AMD and were reduced after anti-VEGF treatment. The PCT of exudative AMD showed no difference after anti-VEGF treatment. Inferior PCT was thinner than all other quadrants in both PCV and exudative AMD before and after anti-VEGF treatment. The initial mean PCT was 1.73-fold thicker in PCV than it was for exudative AMD. These differences decreased by 1.53-fold after anti-VEGF treatment. The mean PCT of PCV decreased by 12.75% after anti-VEGF treatment (p < 0.001), while that of exudative AMD decreased 2.14% (p = 0.392). These results suggest that choroidal thickening in eyes with PCV is not a localized phenomenon limited to the macular area, but is rather a general characteristic extending to the peripapillary area outside the macula.
The blood supply of the choroid region is complicated and can be influenced by many local factors. The vortex vein, ciliary vascular supply, and any bypasses of peripapillary circulation also have an effect on choroidal blood supply. However, the choroid is supplied by various capillary arteries in a relatively distinctive pattern. The nasal choroid is fed by the medial posterior ciliary artery (PCA), whereas the lateral PCA supplies the area of the choroid not supplied by the medial PCA [16]. The posterior choriocapillaris in the peripapillary and submacular regions are fed by short PCAs. The PCA from the ophthalmic artery supplies the choroid around the optic nerve head and also supplies the choroid up to the equator [16]. Usually, the temporal half of the choroid is supplied by the lateral PCA, and the nasal half of the choroid is supplied by the medial PCA [16]. The branches of the PCA consist of long PCAs and short PCAs. The temporal short PCAs, which arise from the lateral PCA, enter the eyeball in the macular region and supply the macular choroid [16]. Because there are no anastomoses between the long PCAs and the short PCAs, there are watershed zones between the areas that are supplied by both [16171819]. They have a segmental distribution without anastomoses and supply a well-defined sector of the choroid [171920].
Our results suggest that the pathophysiology of PCV is related to the entire blood supply to the choroid, rather than being confined only to the focal choroidal vascular abnormality. In addition, the pathogenesis of exudative AMD may be relatively restricted to the macular area and focal chorioretinal abnormalities because thickening of the choroid may be associated with the anatomy and circulatory characteristics of the eye [212223]. The choroid in the macula is somewhat different from the choroid of other areas. The blood supply to the choroid of the macula has two origins involving branches of the short PCAs and a recurrent branch of the long PCA [24]. The presence of very short PCAs selectively directed to the macular region, has been confirmed by previous studies [24]. Very short PCAs may contribute to subfoveal CT and are thought to be related with the development of PCV.
Our finding that the CT decreases in the peripapillary region after anti-VEGF treatment may be another indication of the diffuse choroidal abnormality of PCV. Compared to exudative AMD, PCV has been reported to occur in patients with thick subfoveal choroids [1112]. Yamazaki et al. [25] examined the changes in subfoveal CT after intravitreal injections of ranibizumab for unilateral exudative AMD and PCV. Their results suggested that intravitreal injections of ranibizumab have a pharmacological effect not only on the neovascular lesion, but also on the thickened underlying choroid. Other studies have also reported that an abnormally thickened choroid decreased after anti-VEGF treatment [2627]. In contrast to PCV, PCT of exudative AMD did not change after anti-VEGF treatment, suggesting that diffuse choroidal thickening is associated with the pathogenesis of PCV, and that choroidal thinning, such as vortex decompression, may help to reduce polyp development.
Our results confirmed the asymmetrical distribution of the PCT. The mean PCT showed regional differences and was thickest in the superior region and thinnest in the inferior region in both PCV and exudative AMD. These results are similar to previous OCT studies in normal eyes, which have consistently shown the inferior region to be thinner than other regions [282930]. The thinner choroid in the inferior region makes this area more vulnerable to retinal and choroidal disorders. Several studies have hypothesized that the thinnest peripapillary choroid in the inferior quadrant may involve an area of lower blood supply that may predispose the inferior region of the optic nerve to glaucomatous ischemic damage, supporting a possible explanation for the well-known observation that glaucoma typically affects the inferior optic nerve region first [2831].
A recent study compared changes in subfoveal and peripapillary CT after intravitreal aflibercept or ranibizumab injections in 54 treatment-naïve patients with exudative AMD [32]. They discovered intravitreally injected aflibercept significantly decreased subfoveal CT more than ranibizumab. Additionally, choroidal thinning after aflibercept injection was not limited to the subfoveal area, but instead extended beyond the macula to the peripapillary region. However, we could not find any differences between bevacizumab and ranibizumab. The shape, molecular weight, or mechanism of the antibody may affect the power to reduce CT. Therefore, the effects of different types of anti-VEGF drugs should be evaluated in future studies.
To the best of our knowledge, this is the first study to investigate the PCT in PCV and exudative AMD and the effects of anti-VEGF treatment in the peripapillary region. However, this study had several limitations. First, it was a retrospective study; a comparison with a normal control group was not conducted, and diurnal variations of the CT were not considered [33]. Second, although we compared two groups adjusted for age, the baseline characteristics and types and numbers of anti-VEGF treatments were not controlled. Third, because the peripapillary choroid was thin and the CT measurement was performed manually, the accuracy of measurements was limited. Fourth, previous studies on choroidal hyperpermeability in PCV eyes reported that choroidal thickening might depend on choroidal vascular hyperpermeability [10]. Additional study will be needed to investigate the relationship between increased CT and choroidal hyperpermeability.
In conclusion, eyes with PCV exhibited a thick choroid overall in both the peripapillary and macula regions. Both regions decreased in thickness after anti-VEGF treatment in PCV but not in eyes with exudative AMD. In eyes with exudative AMD, subfoveal CT decreased, but the peripapillary region did not. The results of this study increased our understanding of the pathogenesis of PCV and may provide a basis for the development of new treatments for this disorder.
Figures and Tables
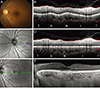 | Fig. 1Measurement of peripapillary choroidal thickness and subfoveal choroidal thickness. (A) Fundus photography of a 56-year-old female with polypoidal choroidal vasculopathy. (B,D) We measured the peripapillary choroidal thickness by subtracting the retinal thickness (D) from the chorioretinal thickness obtained by manual modification of the retinal pigment epithelium line (B). (C) A 360° 3.4-mm-diameter circle scan around the disc was performed to obtain the retinal and choroidal thickness. (E) Horizontal scan line of fundus. (F) Subfoveal choroidal thickness. ILM = internal limiting membrane; SC = sclerochoroidal junction; T = temporal; S = superior, N = nasal; I = inferior; BM = Bruch's membrane. |
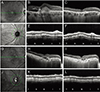 | Fig. 2A representative case of the change in peripapillary choroidal thickness (PCT) following administration of anti-vascular endothelial growth factor (VEGF) in polypoidal choroidal vasculopathy (PCV) and exudative age-related macular degeneration (AMD). (A-F) A 70-year-old female with PCV. (B,C) The subfoveal choroidal thickness decreased from 407 µm to 303 µm. (E,F) The mean PCT was 198 µm and decreased to 150 µm after anti-VEGF administration (25%). (G-L) A 77-year-old female with exudative AMD. (H,I) The subfoveal choroidal thickness decreased from 190 µm to 175 µm (K,L). The mean PCT values were 119 µm before and 114 µm after anti-VEGF. |
 | Fig. 3Change in peripapillary choroidal thickness (PCT) in polypoidal choroidal vasculopathy (PCV) and exudative age-related macular degeneration (AMD). The PCT of PCV was thicker than that of exudative AMD both before and after anti-vascular endothelial growth factor (VEGF) was administered. PCT decreased after anti-VEGF in PCV (*p < 0.022) but not in exudative AMD. Inferior PCT was thinnest in both PCV and exudative AMD both before and after anti-VEGF. †p < 0.018 with post hoc analysis between the sectors of PCV before treatment; ‡p < 0.014 with post hoc analysis between the sectors of exudative AMD before anti-VEGF. |
Acknowledgements
HJK was a consultant/advisor for Allergan, Bayer, and Novartis Pharmaceuticals Corporation. All remaining authors have declared no conflict of interest.
Notes
References
1. Spaide RF, Yannuzzi LA, Slakter JS, et al. Indocyanine green videoangiography of idiopathic polypoidal choroidal vasculopathy. Retina. 1995; 15:100–110.
2. Kuo JZ, Wong TY, Ong FS. Genetic risk, ethnic variations and pharmacogenetic biomarkers in age-related macular degeneration and polypoidal choroidal vasculopathy. Expert Rev Ophthalmol. 2013; 8:127–140.
3. Imamura Y, Engelbert M, Iida T, et al. Polypoidal choroidal vasculopathy: a review. Surv Ophthalmol. 2010; 55:501–515.
4. Laude A, Cackett PD, Vithana EN, et al. Polypoidal choroidal vasculopathy and neovascular age-related macular degeneration: same or different disease? Prog Retin Eye Res. 2010; 29:19–29.
5. Wong RL, Lai TY. Polypoidal choroidal vasculopathy: an update on therapeutic approaches. J Ophthalmic Vis Res. 2013; 8:359–371.
6. Yannuzzi LA, Sorenson J, Spaide RF, Lipson B. Idiopathic polypoidal choroidal vasculopathy (IPCV). Retina. 1990; 10:1–8.
7. Koizumi H, Yamagishi T, Yamazaki T, et al. Subfoveal choroidal thickness in typical age-related macular degeneration and polypoidal choroidal vasculopathy. Graefes Arch Clin Exp Ophthalmol. 2011; 249:1123–1128.
8. Jirarattanasopa P, Ooto S, Nakata I, et al. Choroidal thickness, vascular hyperpermeability, and complement factor H in age-related macular degeneration and polypoidal choroidal vasculopathy. Invest Ophthalmol Vis Sci. 2012; 53:3663–3672.
9. Chung SE, Kang SW, Lee JH, Kim YT. Choroidal thickness in polypoidal choroidal vasculopathy and exudative age-related macular degeneration. Ophthalmology. 2011; 118:840–845.
10. Koizumi H, Yamagishi T, Yamazaki T, Kinoshita S. Relationship between clinical characteristics of polypoidal choroidal vasculopathy and choroidal vascular hyperpermeability. Am J Ophthalmol. 2013; 155:305–313.e1.
11. Warrow DJ, Hoang QV, Freund KB. Pachychoroid pigment epitheliopathy. Retina. 2013; 33:1659–1672.
12. Pang CE, Freund KB. Pachychoroid neovasculopathy. Retina. 2015; 35:1–9.
13. Seddon JM, Sharma S, Adelman RA. Evaluation of the clinical age-related maculopathy staging system. Ophthalmology. 2006; 113:260–266.
14. Oh J, Yoo C, Yun CM, et al. Simplified method to measure the peripapillary choroidal thickness using three-dimensional optical coherence tomography. Korean J Ophthalmol. 2013; 27:172–177.
15. Yiu G, Pecen P, Sarin N, et al. Characterization of the choroid-scleral junction and suprachoroidal layer in healthy individuals on enhanced-depth imaging optical coherence tomography. JAMA Ophthalmol. 2014; 132:174–181.
16. Hayreh SS. Posterior ciliary artery circulation in health and disease: the Weisenfeld lecture. Invest Ophthalmol Vis Sci. 2004; 45:749–757.
17. Hayreh SS. In vivo choroidal circulation and its watershed zones. Eye (Lond). 1990; 4(Pt 2):273–289.
18. Hayreh SS. Inter-individual variation in blood supply of the optic nerve head: its importance in various ischemic disorders of the optic nerve head, and glaucoma, low-tension glaucoma and allied disorders. Doc Ophthalmol. 1985; 59:217–246.
19. Hayreh SS. Segmental nature of the choroidal vasculature. Br J Ophthalmol. 1975; 59:631–648.
20. Hayreh SS. Physiological anatomy of the choroidal vascular bed. Int Ophthalmol. 1983; 6:85–93.
21. Ozcimen M, Sakarya Y, Kurtipek E, et al. Peripapillary choroidal thickness in patients with chronic obstructive pulmonary disease. Cutan Ocul Toxicol. 2016; 35:26–30.
22. Van Keer K, Abegao Pinto L, Willekens K, et al. Correlation between peripapillary choroidal thickness and retinal vessel oxygen saturation in young healthy individuals and glaucoma patients. Invest Ophthalmol Vis Sci. 2015; 56:3758–3762.
23. Fard MA, Abdi P, Kasaei A, et al. Peripapillary choroidal thickness in nonarteritic anterior ischemic optic neuropathy. Invest Ophthalmol Vis Sci. 2015; 56:3027–3033.
24. Yannuzzi LA, Flower RW, Slakter JS, editors. Indocyanine green angiography. St. Louis: Mosby;1997. p. 204.
25. Yamazaki T, Koizumi H, Yamagishi T, Kinoshita S. Subfoveal choroidal thickness after ranibizumab therapy for neovascular age-related macular degeneration: 12-month results. Ophthalmology. 2012; 119:1621–1627.
26. Ahn SJ, Park KH, Woo SJ. Subfoveal choroidal thickness changes following anti-vascular endothelial growth factor therapy in myopic choroidal neovascularization. Invest Ophthalmol Vis Sci. 2015; 56:5794–5800.
27. Razavi S, Souied EH, Darvizeh F, Querques G. Assessment of choroidal topographic changes by swept-source optical coherence tomography after intravitreal ranibizumab for exudative age-related macular degeneration. Am J Ophthalmol. 2015; 160:1006–1013.
28. Ho J, Branchini L, Regatieri C, et al. Analysis of normal peripapillary choroidal thickness via spectral domain optical coherence tomography. Ophthalmology. 2011; 118:2001–2007.
29. Huang W, Wang W, Zhou M, et al. Peripapillary choroidal thickness in healthy Chinese subjects. BMC Ophthalmol. 2013; 13:23.
30. Tanabe H, Ito Y, Terasaki H. Choroid is thinner in inferior region of optic disks of normal eyes. Retina. 2012; 32:134–139.
31. Schwartz B, Harris A, Takamoto T, et al. Regional differences in optic disc and retinal circulation. Acta Ophthalmol Scand. 2000; 78:627–631.
32. Yun C, Oh J, Ahn J, et al. Comparison of intravitreal aflibercept and ranibizumab injections on subfoveal and peripapillary choroidal thickness in eyes with neovascular age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 2016; 254:1693–1702.
33. Tan CS, Ouyang Y, Ruiz H, Sadda SR. Diurnal variation of choroidal thickness in normal, healthy subjects measured by spectral domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2012; 53:261–266.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download