Abstract
Purpose
To investigate the additive effect of oral steroid with topical nonsteroidal anti-inflammatory drug (NSAID) on cystoid macular edema (CME) in patients with epiretinal membrane (ERM) after cataract surgery.
Methods
Medical records of subjects who underwent uneventful cataract surgery (n = 1,349) were retrospectively reviewed; among these patients, those with pre-existing ERM (n = 81) were included. Patients were divided into two groups: one group had postoperative administration of oral steroid for 1 week (n = 45) and the other group did not have oral steroid administration (n = 36). Changes in macular thickness and incidence of CME were compared in both groups. Topical NSAIDs were administered in both groups for 1 month postoperatively. Definite CME and probable CME were defined by changes in retinal contour with or without cystoid changes. Change in central macular thickness of more than three standard deviations (≥90.17 µm) was defined as possible CME. Macular thickness was measured at 1 month after the operation by optical coherence tomography.
Results
The incidence of definite, probable, and possible CME were 2.22%, 4.44%, and 8.89% with the use of steroid and 2.78%, 5.56%, and 8.33% without steroid, respectively (p = 0.694, p = 0.603, and p = 0.625), and regardless of treatment group, the incidences in these patients were higher compared to incidences in whole subjects (1.26%, 2.30%, and 4.32%; p = 0.048, p = 0.032, and p = 0.038, respectively). The differences in macular thickness were not statistically different between the two groups. Average changes of central foveal thickness in 3 mm and 6 mm zone were 29.29 µm, 35.93 µm, and 38.02 µm with the use of steroid and 32.25 µm, 44.08 µm, and 45.39 µm without steroid (p = 0.747, p = 0.148, and p = 0.077, respectively).
Pseudophakic cystoid macular edema (CME), also known as Irvine-Gass syndrome, is the most common cause of unexpected visual loss after cataract surgery [123]. Stress during cataract surgery is thought to trigger an inf lammatory response, resulting in a cascade of inflammatory reactions [123]. This leads to release of free arachidonic acid and prostaglandins, ultimately resulting in breakdown of the blood-retinal barrier and accumulation of intraretinal fluid [4]. Signs and symptoms of CME are generally considered to develop within 4 to 6 weeks after surgery, and may cause temporary or permanent loss of best-corrected visual acuity [5]. To detect CME and evaluate macular thickness, optical coherence tomography (OCT) is frequently used with the increased accuracy of macular thickness measurement [678910]. There have been various efforts to prevent CME after cataract surgery. Topical nonsteroidal anti-inflammatory drugs (NSAIDs), which are known to inhibit cyclooxygenase-1 or cyclooxygenase-2 or both, suppress the release of prostaglandins, and are thus suggested to be beneficial in the treatment and prevention of CME [1112131415161718]. However, even with postoperative topical NSAID use, CME occurs after cataract surgery in patients, especially when pre-existing risk factors for pseudophakic CME (epiretinal membrane [ERM], active choroidal neovascularization, diabetic retinopathy, active uveitis, scleritis, retinal vein occlusion) exist [5192021]. Intravitreal injection of steroid has been used postoperatively in patients with pseudophakic CME; these studies reported decreases in macular thickness and eventual resolution of most of pseudophakic CME [222324]. Posterior subtenon steroid injection has also proven to be effective in pseudophakic CME [2526]. However, considering the complicated procedure and possible side effects such as pain and infection by intravitreal or posterior subtenon steroid injection, we suggest use of postoperative oral steroids as prophylactic medications for preventing pseudophakic CME in patients who have pre-existing risk factors. Systemic corticosteroid is a widely used anti-inflammatory medication in various ocular diseases [2728]. However, the effect of oral steroid in preventing pseudophakic CME has not been well established in previous studies. Since April 2013, additional oral steroids were prescribed in patients with pre-existing risk factors for pseudophakic CME after cataract surgery to investigate the additional preventive effect. This study was designed to evaluate the additive effect of oral steroids in preventing pseudophakic CME in patients with pre-existing ERM after phacoemulsification and intraocular lens (IOL) implantation.
This study was a retrospective comparative clinical study. The institutional review board of Seoul National University Hospital approved the study protocol (No. 1401-093-549), and the protocol complied with the tenets of the Declaration of Helsinki. Medical records of subjects who underwent uneventful phacoemulsification and IOL implantation (n = 1,349) at the Department of Ophthalmology of Seoul National University Hospital from January 2011 to December 2013 were retrospectively reviewed. Among these patients, 81 eyes of 81 subjects who had pre-existing ERM were enrolled. From January 2011 to March 2012, patients used topical NSAIDs postoperatively without oral steroid, and from April 2012 to December 2013, patients used topical NSAIDs with additional oral steroid postoperatively. All subjects had been followed up for at least 1 month. Exclusion criteria were as follows: subjects with macular edema present preoperatively, active choroidal neovascularization with/without intravitreal anti-vascular endothelial growth factor antibody/steroid injection history, previously vitrectomized eyes, other risk factors for pseudophakic CME such as diabetic retinopathy, active uveitis/scleritis, retinal vein occlusion, and those unable to retrieve preoperative images of OCT measurements due to severe lens opacity were excluded. Subjects who had contraindications to oral steroid were also excluded.
Cataract surgery was performed using the standard technique by one experienced surgeon (MKK). After clear corneal incision, capsulorhexis was performed by curved needle and forceps with DisCoVisc (sodium hyaluronate and sodium chondroitin sulfate; Alcon, Fort Worth, TX, USA), then followed by the phaco-chop technique phacoemulsification method. Hydrophobic acrylic posterior chamber-IOL was implanted ‘in the bag’ in all subjects. The viscoelastic material was removed carefully from the anterior chamber and from behind the posterior chamber-IOL. All subjects were treated with topical 0.1% diclofenac (Diclan; Hanlim, Seoul, Korea) four times a day for 8 weeks, 1% prednisolone (Pred Forte; Allergan, Dublin, Ireland) four times a day for 4 weeks, and 0.5% moxifloxacin (Vigamox, Alcon) four times a day for 4 weeks, postoperatively. All subjects were prescribed aceclofenac (Asec; Hanmi, Seoul, Korea) 100 mg twice a day for 3 days postoperatively. In the topical NSAID with oral steroid use group, oral prednisolone acetate (Solondo; Yuhan, Seoul, Korea) was additionally prescribed 30 mg once a day for 7 days postoperatively.
The main outcome measurement was as follows: (1) incidence of CME, including definite and probable CME, in the topical NSAID with oral steroid use group (group 1) and topical NSAID only group (group 2), (2) topographic analysis of macular thickness change in the topical NSAID with oral steroid use group and topical NSAID only group.
The Cirrus HD-OCT model 4000 (Carl Zeiss Meditec, Dublin, CA, USA) was used to identify pre-existing ERM, measure macular thickness, and detect macular edema. OCT images were retrieved before and 4 weeks after surgery. Pupils were dilated for OCT examination in all cases with 0.5% tropicamide/phenylephrine (Tropherine, Hanmi). Macular thickness was measured in the fovea and perifoveal zones (fovea +3 mm, fovea +6 mm ring quadrants) according to the regions determined in the Early Treatment Diabetic Retinopathy Study. Thickness values of the following macular regions were calculated: foveal value (F), the average of fovea +3 mm perifoveal ring (F +3 mm, including 5 zones), average of 3 mm perifoveal ring (3 mm, including 4 zones), fovea +6 mm perifoveal ring (F +6 mm, including 9 zones), and average of 6 mm perifoveal ring (6 mm, including 8 zones) (Fig. 1) [10]. The OCT measurement was implemented along six radial scans of 6 mm in length centered on the foveola. The macular thickness map was derived using data from the six radial scans.
The anterior segment was examined using slit lamp microscopy (BQ 900; Haag-streit diagnostics, Koeniz, Switzerland) and the fundus was examined using indirect ophthalmoscopy (Vantage Plus; Keeler, Broomall, PA, USA) before and after surgery.
Changes in macular thickness were defined based on the OCT-findings as follows. (1) Definite CME: presence of cystoid changes associated with substantial (≥40 µm) retinal thickening evident on OCT (Fig. 2A, 2B) [17]. (2) Probable CME: presence of changes in retinal contour and increased macular thickness relative to preoperative baseline, but without definite cystoid changes (Fig. 2C, 2D) [17]. (3) Possible CME: presence of changes in central macular thickness (CMT) of more than three standard deviations (≥90.17 µm) without definite cystoid changes or changes in retinal contour (Fig. 2E, 2F) [610].
We analyzed demographic factors, incidence of definite/probable or possible CME and compared changes in macular thickness between the topical NSAID with oral steroid use group and topical NSAID only group. The average grading of nuclear sclerosis (Lens Opacities Classification system, version III) of subjects was evaluated in both groups.
All data was analyzed using IBM SPSS Statistics ver. 21.0 (IBM Corp., Armonk, NY, USA). To compare the baseline characteristics of the two groups and to compare preoperative CMT with postoperative CMT, statistical significance was analyzed using the chi-square test, independent t-test, Welch t-test, and Fisher exact test. The level of significance was set at 0.05.
Demographic data of the subjects enrolled in this study are shown in Table 1. Thirty-three subjects (73.33%) were female in group 1; 20 subjects (55.56%) were female in group 2. The mean age was 69.00 ± 9.34 and 67.33 ± 12.23 years in group 1 and group 2, respectively; gender and mean age were not statistically different between the two groups (p = 0.076 and p = 0.179, respectively) (Table 1). Ten patients (22.22%) had diabetes mellitus in group 1, and 13 subjects (36.11%) had diabetes mellitus in group 2, which was not statistically different (p = 0.129) (Table 1). The average grading of nuclear sclerosis was not different between the two groups (p = 0.856) (Table 1). No side effect associated with the use of oral steroid was observed.
First, we evaluated the changes in CMT before surgery and 1 month after surgery. Mean CMT of total subjects was 276.00 µm in group 1 and 280.36 µm in group 2 preoperatively. Mean CMT of all subjects was 305.29 µm in group 1 and 312.61 µm in group 2 postoperatively (Table 2). Mean macular thickness change in the central foveal area was 29.29 µm in group 1 and 32.25 µm in group 2 (Table 2). Mean perifoveal macular thickness changes in the 3 mm area and 6 mm area were 35.93 µm and 38.02 µm, respectively in group 1 and 44.08 µm and 45.39 µm, respectively in group 2 (Table 2). Topographic analysis of changes in macular thickness showed insignif icant differences in central foveal area, perifoveal 3 mm area, and perifoveal 6 mm area between two groups.
Next, we examined the incidence of CME in both groups. In group 1, the incidences of definite, probable, and possible CME were 2.22% (one eye), 4.44% (two eyes), and 8.89% (four eyes), respectively. In group 2, the incidences of definite, probable, and possible CME were 2.78% (one eye), 5.56% (two eyes), and 8.33% (three eyes), respectively (Table 3). These incidences were not statistically different between the two groups (p = 0.694, p = 0.603, and p = 0.625, respectively). Analyses revealed that clinically relevant CME (definite + probable CME) developed in 6.67% of patients in group 1 and 8.33% of patients in group 2; meanwhile, possible CME developed in 8.89% of patients in group 1 and 8.33% of patients in group 2 (Table 3).
Results from this study determined that the incidence of clinically relevant CME (definite + probable CME) and possible CME af ter phacoemulsif ication and IOL implantation with pre-existing ERM was 7.41% and 8.64%, respectively. Few studies have reported the incidence of macular edema in patients with pre-existing ERM. Henderson et al. [5] reported that the incidence of pseudophakic CME with pre-existing ERM was 7.7%, which was comparable with the incidence measured in our study. However, the quantitative diagnostic criteria for CME was not specified in the previous study; therefore, direct comparison with our result is somewhat difficult.
Giansanti et al. [8] previously reported that a statistically significant increase in CMT was observed from day 30 in patients with ERM. The results from the present study showed that changes in macular thickness (central foveal area, perifoveal 3 mm area, and perifoveal 6 mm area) and the incidence of CME were not statistically different between the oral steroid with topical NSAIDs group and topical NSAIDs only group. This result suggests that oral steroids may not have additive effects on changes in retinal thickness in patients with pre-existing ERM af ter phacoemulsification and IOL implantation, and may not prevent clinically significant CME.
In a total 1,349 patients who underwent uneventful cataract surgery from January 2011 to December 2013, the incidences of definite, probable, and possible CME were 1.26% (17 eyes), 2.30% (32 eyes), and 4.32% (eyes), respectively, which were all statistically lower than the incidences in patients with pre-existing ERM, regardless of the use of oral steroid (p = 0.048, p = 0.032, and p = 0.038 respectively). This result suggests that pre-existing ERM is a risk factor for pseudophakic CME, which is consistent with previous studies [526]. Henderson et al. [5] reported that the blood-retinal barrier is compromised in patients with ERM, and multiple insults to vascular permeability could increase the risk for postoperative macular edema. Similarly, w e h ypothesized t hat t raction forces on the macula or surrounding area may increase the risk of breakdown in the blood-aqueous barrier, inf luence vascular permeability, and increase the incidence of pseudophakic CME. Inflammatory mediators concerned in ERM formation might also cause macular edema in the postoperative period.
Topical NSAIDs have been shown to be effective in preventing pseudophakic CME, and recently topical NSAIDs are frequently used after cataract surgery [13141516172429]. However, even with the use of NSAIDs, CME f requently occurred after cataract surgery when patients have risk factors for pseudophakic CME, so we investigated the additive effect of oral steroid in preventing pseudophakic CME. The pathogenesis of pseudophakic CME is thought to be multifactorial. The major etiology appears to be inflammatory mediators that are upregulated in the aqueous and vitreous humors after surgical manipulation [2430]. Inflammation breaks down the blood-aqueous and blood-retinal-barriers, which leads to increased vascular permeability [2430]. Steroids are known to stabilize the blood-retinal barrier, resorption of exudation, and downregulation of inflammatory stimuli, which may reduce macular edema [31]. In this study, no complications were found in patients who were prescribed oral steroid. However, the additive effects of oral steroids in preventing CME were not statistically significant. This could be due to several reasons. Both groups were already treated with topical steroids for 4 weeks and additional oral steroids might not have additive effects. On the other hand, the amount of oral steroid used may not have been enough to prevent inflammation. In our study, patients in the oral steroid use group were prescribed oral prednisolone 30 mg once a day for 7 days postoperatively and discontinued. The prophylactic dose of oral steroid in uveitis patients was 0.5 mg/kg/day for 2 weeks in a previous study and in the present study we used the same dose of oral steroids for patients with pre-existing risk factors of pseudophakic CME [32]. The average weight of subjects receiving cataract surgery was close to 60 kg and we determined that a dose of 30 mg per day was appropriate. Considering the possible systemic side effects, we decided on a duration of oral steroid use of 1 week and recommended discontinuation afterwards. Further studies with increased doses of oral steroid, or increased duration of oral steroid use are needed to confirm our results.
Our findings should be understood within the limitations of the study. First, we did not compare ultrasound energy dissipation, amount of irrigation fluid, manipulations, and occlusion break response in patients. These intraoperative surgical factors might affect the postoperative macular thickness and the incidence of CME [2]. We supposed that the degree of nuclear sclerosis might be proportional to the ultrasound energy dissipation, and we only compared the average degree of nuclear sclerosis in patients between the two groups. Second, we only investigated patients with pre-existing ERM. Future studies including patients with other risk factors for pseudophakic CME such as diabetic retinopathy, active uveitis, scleritis, or retinal vein occlusion are needed to evaluate the effect of oral steroids on preventing pseudophakic CME. Moreover, rebound reaction to sudden discontinuation of steroids might occur in patients, offsetting the additive effect of oral steroids in preventing CME. Further studies with slow tapering of steroids would reveal the rebound effects of steroids.
In conclusion, the administration of oral steroids may not have an additive effect on preventing pseudophakic CME and retinal thickness in patients with pre-existing ERM after phacoemulsification and IOL implantation, when topical NSAIDs are applied. Considering the limitations of the present study, further prospective studies are needed to confirm the results from our study.
Figures and Tables
Fig. 1
The distribution of central macular thickness based on optical coherence tomography analysis is illustrated.

Fig. 2
(A-F) Optical coherence tomography images of cystoid macular edema (CME). (A,B) Preoperative and postoperative findings of definite CME. (C,D) Preoperative and postoperative findings of probable CME. (E,F) Preoperative and postoperative findings of possible CME.
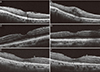
Table 1
Baseline demographic data of the topical NSAID with oral steroid group and topical NSAID only group

References
1. Irvine SR. A newly defined vitreous syndrome following cataract surgery. Am J Ophthalmol. 1953; 36:599–619.
2. Flach AJ. The incidence, pathogenesis and treatment of cystoid macular edema following cataract surgery. Trans Am Ophthalmol Soc. 1998; 96:557–634.
3. Yonekawa Y, Kim IK. Pseudophakic cystoid macular edema. Curr Opin Ophthalmol. 2012; 23:26–32.
4. Gass JD, Norton EW. Cystoid macular edema and papilledema following cataract extraction: a fluorescein fundoscopic and angiographic study. Arch Ophthalmol. 1966; 76:646–661.
5. Henderson BA, Kim JY, Ament CS, et al. Clinical pseudophakic cystoid macular edema: risk factors for development and duration after treatment. J Cataract Refract Surg. 2007; 33:1550–1558.
6. Kusbeci T, Eryigit L, Yavas G, Inan UU. Evaluation of cystoid macular edema using optical coherence tomography and fundus fluorescein angiography after uncomplicated phacoemulsification surgery. Curr Eye Res. 2012; 37:327–333.
7. Perente I, Utine CA, Ozturker C, et al. Evaluation of macular changes after uncomplicated phacoemulsification surgery by optical coherence tomography. Curr Eye Res. 2007; 32:241–247.
8. Giansanti F, Bitossi A, Giacomelli G, et al. Evaluation of macular thickness after uncomplicated cataract surgery using optical coherence tomography. Eur J Ophthalmol. 2013; 23:751–756.
9. Kim SJ, Belair ML, Bressler NM, et al. A method of reporting macular edema after cataract surgery using optical coherence tomography. Retina. 2008; 28:870–876.
10. Biro Z, Balla Z. OCT measurements on the foveal and perifoveal retinal thickness on diabetic patients af ter phacoemulsification and IOL implantation. Eye (Lond). 2010; 24:639–647.
11. Shimura M, Nakazawa T, Yasuda K, Nishida K. Diclofenac prevents an early event of macular thickening after cataract surgery in patients with diabetes. J Ocul Pharmacol Ther. 2007; 23:284–291.
12. Miyake K, Nishimura K, Harino S, et al. The effect of topical diclofenac on choroidal blood flow in early postoperative pseudophakias with regard to cystoid macular edema formation. Invest Ophthalmol Vis Sci. 2007; 48:5647–5652.
13. O'Brien TP. Emerging guidelines for use of NSAID therapy to optimize cataract surgery patient care. Curr Med Res Opin. 2005; 21:1131–1137.
14. Wolf EJ, Braunstein A, Shih C, Braunstein RE. Incidence of visually significant pseudophakic macular edema after uneventful phacoemulsification in patients treated with nepafenac. J Cataract Refract Surg. 2007; 33:1546–1549.
15. Miyake K, Ota I, Miyake G, Numaga J. Nepafenac 0.1% versus fluorometholone 0.1% for preventing cystoid macular edema after cataract surgery. J Cataract Refract Surg. 2011; 37:1581–1588.
16. Elsawy MF, Badawi N, Khairy HA. Prophylactic postoperative ketorolac improves outcomes in diabetic patients assigned for cataract surgery. Clin Ophthalmol. 2013; 7:1245–1249.
17. Wittpenn JR, Silverstein S, Heier J, et al. A randomized, masked comparison of topical ketorolac 0.4% plus steroid vs steroid alone in low-risk cataract surgery patients. Am J Ophthalmol. 2008; 146:554–560.
18. Donnenfeld ED, Nichamin LD, Hardten DR, et al. Twice-daily, preservative-free ketorolac 0.45% for treatment of inflammation and pain after cataract surgery. Am J Ophthalmol. 2011; 151:420–426.e1.
19. Shah AS, Chen SH. Cataract surgery and diabetes. Curr Opin Ophthalmol. 2010; 21:4–9.
20. Sijssens KM, Los LI, Rothova A, et al. Long-term ocular complications in aphakic versus pseudophakic eyes of children with juvenile idiopathic arthritis-associated uveitis. Br J Ophthalmol. 2010; 94:1145–1149.
21. Rashid S, Young LH. Progression of diabetic retinopathy and maculopathy after phacoemulsification surgery. Int Ophthalmol Clin. 2010; 50:155–166.
22. Williams GA, Haller JA, Kuppermann BD, et al. Dexamethasone posterior-segment drug delivery system in the treatment of macular edema resulting from uveitis or Irvine-Gass syndrome. Am J Ophthalmol. 2009; 147:1048–1054. 1054.e1–1054.e2.
23. Jonas JB, Kreissig I, Degenring RF. Intravitreal triamcinolone acetonide for pseudophakic cystoid macular edema. Am J Ophthalmol. 2003; 136:384–386.
24. Shelsta HN, Jampol LM. Pharmacologic therapy of pseudophakic cystoid macular edema: 2010 update. Retina. 2011; 31:4–12.
25. Yuksel B, Uzunel UD, Kerci SG, et al. Comparison of subtenon triamcinolone acetonide injection with topical nepafenac for the treatment of pseudophakic cystoid macular edema. Ocul Immunol Inflamm. 2016; 1–7.
26. Guo S, Patel S, Baumrind B, et al. Management of pseudophakic cystoid macular edema. Surv Ophthalmol. 2015; 60:123–137.
27. Stanford MR, Verity DH. Diagnostic and therapeutic approach to patients with retinal vasculitis. Int Ophthalmol Clin. 2000; 40:69–83.
28. Jabs DA, Rosenbaum JT, Foster CS, et al. Guidelines for the use of immunosuppressive drugs in patients with ocular inflammatory disorders: recommendations of an expert panel. Am J Ophthalmol. 2000; 130:492–513.
29. Heier JS, Topping TM, Baumann W, et al. Ketorolac versus prednisolone versus combination therapy in the treatment of acute pseudophakic cystoid macular edema. Ophthalmology. 2000; 107:2034–2038.
30. Benitah NR, Arroyo JG. Pseudophakic cystoid macular edema. Int Ophthalmol Clin. 2010; 50:139–153.
31. Folkman J, Ingber DE. Angiostatic steroids: method of discovery and mechanism of action. Ann Surg. 1987; 206:374–383.
32. Meacock WR, Spalton DJ, Bender L, et al. Steroid prophylaxis in eyes with uveitis undergoing phacoemulsification. Br J Ophthalmol. 2004; 88:1122–1124.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download