Abstract
Purpose
To compare vascular displacement in the macula after surgical closure of idiopathic macular hole (MH) after single-layered inverted internal limiting membrane (ILM) flap technique and conventional ILM removal.
Methods
This retrospective study included patients who underwent either vitrectomy and ILM removal only or vitrectomy with single-layered inverted ILM flap for idiopathic MH larger than 400 µm from 2012 to 2015. A customized program compared the positions of the retinal vessels in the macula between preoperative and postoperative photographs. En face images of 6 × 6 mm optical coherence tomography volume scans were registered to calculate the scale. Retinal vessel displacement was measured as a vector value by comparing its location in 16 sectors of a grid partitioned into eight sectors in two rings (inner, 2 to 4 mm; outer, 4 to 6 mm). The distance and angle of displacement were calculated as an average vector and were compared between the two groups for whole sectors, inner ring, outer ring, and for each sector.
Results
Twenty patients were included in the ILM flap group and 22 in the ILM removal group. There were no statistical differences between the groups for baseline characteristics. The average displacement in the ILM flap group and the ILM removal group was 56.6 µm at −3.4° and 64.9 µm at −2.7°, respectively, for the whole sectors (p = 0.900), 76.1 µm at −1.1° and 87.3 µm at −0.9° for the inner ring (p = 0.980), and 37.4 µm at −8.2° and 42.7 µm at −6.3° for the outer ring (p = 0.314). There was no statistical difference in the displacement of each of the sectors.
Macular hole (MH) is characterized by a full-thickness defect at the fovea leading to the loss of central vision [1]. The compartment formed by gas tamponade has been suggested to induce glial proliferation and centripetal contraction of the retinal tissue, resulting in hole closure [2]. Removal of the internal limiting membrane (ILM) improves the anatomical success rate by facilitating the aforementioned process [3]. However, ILM peeling is reportedly associated with incidences of surgical trauma [4], dissociated optic nerve fiber layer [5], and macular displacement [67]. In particular, macular displacement was related to the incidence of postoperative metamorphopsia, which has important, functional implications for the quality of life of the patient as well as visual acuity [89].
Meanwhile, closure of MH is still challenging in large, chronic MH or in highly myopic eyes; therefore, diverse techniques have been introduced, including autologous serum and ILM flap technique [1011]. The inverted ILM flap technique uses the ILM for coverage of the MH instead of simply removing the ILM and has a reportedly improved surgical closure rate [11121314]. Although the technique is gaining popularity as an adjuvant procedure for cases related to lower closure rates, postoperative anatomical changes of the macular structures have not been investigated as much as with the conventional procedure. ILM may act as a scaffold for glial cells to proliferate and contribute to the enhancement of the compartment by blocking the entrance of fluid from the vitreous cavity to the MH [12]. Accordingly, the macular displacement after ILM flap procedure can be different from that of conventional ILM removal.
The purpose of the present study was to investigate macular displacement in idiopathic MH after single-layered ILM flap surgery by comparing postoperative vascular displacement in the macula between the flap procedure and the conventional removal.
The current study was a retrospective review of the medical records of patients who had been diagnosed and treated with MH larger than 400 µm at Pusan National University Hospital and Pusan National University Yangsan Hospital from 2012 to 2015.
Patients who had a significant epiretinal membrane, high myopia over −5 diopters, clinically significant cataract that disables the visibility of retinal vessels, or other retinal diseases that can cause bias in the interpretation of vascular displacement were excluded.
The patients were divided into two groups: the ILM removal group and the ILM flap group. The former group underwent vitrectomy combined with conventional removal of the ILM. To enhance visualization of the ILM, triamcinolone acetonide, indocyanine green, or brilliant blue G was used at the surgeon's discretion. The latter group underwent vitrectomy combined with single-layered inverted ILM flap technique as reported previously [13]. In brief, the ILM of approximately one disc area attached to the superior margin of the MH was preserved for a flap after staining the ILM with brilliant blue G. The ILM flap was flipped to cover the MH, and the position was maintained using perfluoro-n-octane on the flap during fluid-air exchange. The perfluoro-n-octane was aspirated at the end of the exchange, and the residual heavy liquid was removed by evaporation without a lavage. Vitrectomy was performed using the Constellation/Accurus System (Alcon Laboratories, Fort Worth, TX, USA), a sutureless 23- or 25-gauge vitrectomy system, by four surgeons (KYP, SWP, ISB, and JEL). ILM was stained by triamcinolone acetonide, indocyanine green, or brilliant blue G. Phacoemulsification was combined concurrently at the surgeon's discretion.
Vascular displacement was analyzed as described in a previous report (Fig. 1A and 1B) [15]. Briefly, a set of fundus photographs were taken using the same fundus camera (AFC 330; Nidek, Gamagori, Japan or CR-2; Canon, Tokyo, Japan) at baseline and at 4 to 6 months postoperatively. A set using a different type of fundus camera and a set of two photographs in which the disc overlapped with a disparity of more than 0.5 disc diameter (DD) were excluded in order to minimize bias caused by image distortion. The retinal vessels were enhanced in the green channel of fundus photograph. Preoperative and postoperative photographs were overlapped by matching three bifurcating points. An en face optical coherence tomography (Cirrus OCT; Carl Zeiss Meditec, Dublin, CA, USA or DRI OCT-1 Atlantis; Topcon, Tokyo, Japan) image of a 6 × 6 mm cube scan was registered to calculate the scale of the photographs.
The macula was partitioned via a customized grid of 16 sectors. The grid was composed of two rings each containing eight sectors: temporal, supero-temporal, superior, supero-nasal, nasal, infero-nasal, inferior, and infero-temporal. The diameters of the inner and outer rings were 2 to 4 mm and 4 to 6 mm, respectively. The area located less than 1 mm from the foveal center was excluded due to vascular paucity.
The vascular displacement was assessed in each sector by comparing the location of the same vascular bifurcation, or distinct landmark, between preoperative and postoperative photographs. If any landmark for measurement was not found in the sector, the displacement was not measured. Vascular displacement was measured as a vector (as the x and y coordinates), and its distance and angle were calculated in the inner and outer rings as well as in each sector. Measurements of the left eye were flipped horizontally to maintain consistency in displacement angle calculations. The angle was defined as 0° in the nasal and horizontal directions and increased in a counterclockwise direction.
The measured baseline characteristics were age, sex, adjuvant, size of the ILM removed, size of the MH, and preoperative best-corrected visual acuity (BCVA). The hole diameter was measured as the minimum diameter between the hole margin in the horizontal B scan of optical coherence tomography. BCVA was quantified using lines of Snellen visual acuity, which was converted to a logarithm of the minimum angle of resolution (logMAR) for statistical analysis. The size of the ILM removal was obtained based on the operation records.
All statistical analyses were performed using SPSS ver. 21.0 (IBM Corp., Armonk, NY, USA). Displacement was analyzed based on the distance of displacement (horizontal, vertical, and length of the vector). Differences between study groups were assessed using Fisher's exact test or the Mann-Whitney U-test. The p-values <0.05 were considered statistically significant.
The ILM flap group consisted of four men and 16 women with a mean age of 65.7 ± 6.1 years (56 to 80 years), while the ILM removal group consisted of five men and 17 women with a mean age of 66.4 ± 5.1 years (59 to 78 years). The preoperative BCVA was 0.99 ± 0.36 logMAR and 0.95 ± 0.27 logMAR, respectively. The minimum diameter of the MH was 600.1 ± 145.0 µm and 600.2 ± 139.6 µm, respectively. In the ILM flap group, the size of the ILM removed was 4 DD in eight eyes, 3.5 DD in two eyes, and 3 DD in eight eyes. In the ILM removal group, the size of the ILM removal was 4 DD in 10 eyes, 3.5 DD in three eyes, and 3 DD in eight eyes. A 25G instrument and brilliant blue G for staining the ILM were used more frequently in the ILM flap group. There were no significant differences in terms of age, sex, preoperative BCVA, diameters of MH and size of ILM removal. The baseline characteristics of each group are summarized in Table 1.
Vascular displacement was measured in 85.0% of the sectors. The superior sector of the inner ring had the highest rate of measurement (97.6%), and the nasal sector of the outer ring had the lowest (64.3%). There was no difference in vascular displacement between the two groups (84.1% for the ILM flap group vs. 85.8% for the ILM peeling group).
In the ILM flap group, the mean displacement was 56.6 µm at −3.4° in the whole sectors, 76.1 µm at −1.1° in the inner ring, and 37.4 µm at −8.2° in the outer ring. Displacement was statistically larger in the inner ring than in the outer ring (p < 0.001). Mean displacement was largest (118.0 µm) in the supero-temporal sector of the inner ring and smallest (22.0 µm) in the infero-nasal sector of the outer ring. In the ILM peeling group, the mean displacement was 64.9 µm at −2.7° in the whole sectors, 87.3 µm at −0.9° in the inner ring, and 42.7 µm at −6.3° in the outer ring. Displacement was statistically larger in the inner ring than in the outer ring (p < 0.001). Mean displacement was largest (126.7 µm) in the supero-temporal sector of the inner ring and smallest (34.5 µm) in the nasal sector of the outer ring.
The vascular displacement was nasally-directed in all sectors. Superior displacement was noted in the inferior sectors, and inferior displacement was noted in the superior sectors. The distance and angle of mean vascular displacement of the ILM flap group and the ILM removal group are summarized in Table 2, Fig. 2A and 2B.
The horizontal, vertical, and distance of displacement were not different between the two groups for the whole sectors, inner ring, outer ring, or for each of the sectors (p = 0.075 to 1.000).
The inverted ILM flap technique, which retains the ILM around the MH, reported higher surgical closure rates than the conventional ILM peeling technique [111314]. The first reported application of this technique was by Michalewska, who covered the MH with folded ILM [11]. We modified this technique for coverage of the MH with a single-layered inverted flap of the ILM with the assistance of perfluoro-n-octane [13]. All patients in the ILM flap group of the present study underwent the single-layered ILM flap procedure. Although the exact mechanism remains unclear, the inverted ILM flap technique appeared to form a long-lasting structural compartment between the MH and the vitreous cavity, facilitating glial cell proliferation, in which the ILM acted as a medium to thereby result in the closure of the MH [1112]. This hypothesis was supported by the presentation of delayed closure and the incidence of localized hyper-proliferation after an inverted flap surgery [1617].
More glial proliferation could affect the postoperative displacement of the macula tissue, and the displacement pattern could differ from that expected following the conventional ILM removal. This study was performed to investigate the vascular displacement in idiopathic MH after single-layered ILM flap surgery. We found that the postoperative topographic changes showed no significant differences between the inverted ILM flap group and the conventional ILM removal group.
Occasionally, patients complain of metamorphopsia despite reporting good visual acuity after a successful operation [9]. Postoperative, topographic changes of the macula seem to be related to postoperative metamorphopsia. The nasal displacement of the fovea and asymmetric elongation of the foveal tissue were correlated with postoperative metamorphopsia [8]. However, measurement of the displacement at several selected points provides limited elucidation of the exact mechanism of topographic changes and their relationship with postoperative metamorphopsia.
The authors investigated retinal vascular displacement to analyze the topographic changes of the macula. This method, using our customized program, analyzed the macula in greater detail compared to previous studies that analyzed the displacement evident in tomographic images by partitioning the fovea horizontally or vertically [671819]. Our method demonstrated high consistency and reproducibility [15]. The multi-center study about topographic changes of the macula after ILM removal revealed asymmetric displacement in the nasal and temporal sectors and proposed that the combined forces of the centripetal contraction of a closing MH, the contraction of the retinal nerve fiber layer, and gravity contributed to the mechanism of displacement [15]. Furthermore, ILM peeling seemed to induce retinal displacement by removal of the rigid support preventing displacement.
Overall, the displacement pattern was also directed nasally and slightly inferiorly, as similarly indicated by a previous report [15]. The mean displacement was not significantly different between the two groups for the whole sectors, inner ring, outer ring, or each of the sectors. This may be accounted for by two explanations. First, the extent of glial proliferation under the ILM flap may have resulted in negligible differences in the mean displacement compared to conventional removal procedures. Second, the area located closer than 1 mm from the foveal center was excluded from the displacement measurement due to vascular paucity. As the size of the ILM flap was usually limited to the area within 1 mm from the foveal center, its effect would also be localized to the area around the fovea. These aspects might affect postoperative metamorphopsia differently and should be investigated in future studies.
Several limitations were noted in this study. This study was retrospectively designed and included a relatively small number of patients. There was difference in baseline characteristics of the surgical instruments and adjuvant for ILM removal. However, bias by these factors should be minimal, as the diameter of a surgical instrument would not affect postoperative displacement, and no differences were found in vascular displacement regarding adjuvant to enhance visualization of the ILM in a previous report [15]. Additionally, the retinal vessels presented displacement of the inner retina and might not reflect the outer retina, and postoperative metamorphopsia was not assessed for its correlative link to postoperative displacement.
In conclusion, postoperative vascular displacement of the macula after single-layered ILM inverted flap surgery in cases of idiopathic MH was directed toward the horizontal raphe, the optic disc, and slightly inferiorly. Additionally, the distance and angle of vascular displacement were not different from those after conventional ILM removal. Further research should be carried out to evaluate postoperative metamorphopsia after ILM flap surgery and its relation to macular displacement in a comparative study.
Figures and Tables
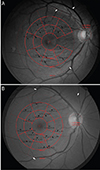 | Fig. 1Analysis of vascular displacement of the macula after single-layered inverted internal limiting membrane flap (A) and conventional internal limiting membrane removal (B) for large idiopathic macular hole using a customized program. Black arrows represent displacement in each sector partitioned by customized grid. Unselected grids indicate excluded sectors owing to lack of a vascular landmark. Red and blue crosses indicate registered points to match fundus photo to optical coherence tomography. Preoperative and postoperative fundus photos were matched by registering the vascular bifurcations (white arrows). |
 | Fig. 2Schematic drawing shows the displacement of the retinal vessels after single-layered inverted internal limiting membrane flap (A) and conventional internal limiting membrane removal (B) for large idiopathic macular hole. Thin arrows represent displacement in each case. Thick arrows represent mean displacement in each sector. Displacement was not measured in the fovea due to the vascular paucity, and the arrows in the foveal center represent average displacement in the whole sectors. The lengths of arrows are enlarged three times than that of the actual displacement to improve the visualization. |
References
1. la Cour M, Friis J. Macular holes: classification, epidemiology, natural history and treatment. Acta Ophthalmol Scand. 2002; 80:579–587.
2. Mester V, Kuhn F. Internal limiting membrane removal in the management of full-thickness macular holes. Am J Ophthalmol. 2000; 129:769–777.
3. Tognetto D, Grandin R, Sanguinetti G, et al. Internal limiting membrane removal during macular hole surgery: results of a multicenter retrospective study. Ophthalmology. 2006; 113:1401–1410.
4. Haritoglou C, Gass CA, Schaumberger M, et al. Macular changes after peeling of the internal limiting membrane in macular hole surgery. Am J Ophthalmol. 2001; 132:363–368.
5. Ito Y, Terasaki H, Takahashi A, et al. Dissociated optic nerve fiber layer appearance after internal limiting membrane peeling for idiopathic macular holes. Ophthalmology. 2005; 112:1415–1420.
6. Ishida M, Ichikawa Y, Higashida R, et al. Retinal displacement toward optic disc after internal limiting membrane peeling for idiopathic macular hole. Am J Ophthalmol. 2014; 157:971–977.
7. Kawano K, Ito Y, Kondo M, et al. Displacement of foveal area toward optic disc after macular hole surgery with internal limiting membrane peeling. Eye (Lond). 2013; 27:871–877.
8. Kim JH, Kang SW, Park DY, et al. Asymmetric elongation of foveal tissue after macular hole surgery and its impact on metamorphopsia. Ophthalmology. 2012; 119:2133–2140.
9. Fukuda S, Okamoto F, Yuasa M, et al. Vision-related quality of life and visual function in patients undergoing vitrectomy, gas tamponade and cataract surgery for macular hole. Br J Ophthalmol. 2009; 93:1595–1599.
10. Konstantinidis A, Hero M, Nanos P, Panos GD. Efficacy of autologous platelets in macular hole surgery. Clin Ophthalmol. 2013; 7:745–750.
11. Michalewska Z, Michalewski J, Adelman RA, Nawrocki J. Inverted internal limiting membrane flap technique for large macular holes. Ophthalmology. 2010; 117:2018–2025.
12. Michalewska Z, Michalewski J, Dulczewska-Cichecka K, et al. Temporal inverted internal limiting membrane flap technique versus classic inverted internal limiting membrane flap technique: a comparative study. Retina. 2015; 35:1844–1850.
13. Shin MK, Park KH, Park SW, et al. Perfluoro-n-octane-assisted single-layered inverted internal limiting membrane flap technique for macular hole surgery. Retina. 2014; 34:1905–1910.
14. Michalewska Z, Michalewski J, Dulczewska-Cichecka K, Nawrocki J. Inverted internal limiting membrane flap technique for surgical repair of myopic macular holes. Retina. 2014; 34:664–669.
15. Pak KY, Park KH, Kim KH, et al. Topographic changes of the macula after closure of idiopathic macular hole. Retina. 2017; 37:667–672.
16. Chung CY, Wong DS, Li KK. Is it necessary to cover the macular hole with the inverted internal limiting membrane flap in macular hole surgery? A case report. BMC Ophthalmol. 2015; 15:115.
17. Okuda T, Higashide T, Kobayashi K, et al. Macular hole closure over residual subretinal fluid by an inverted internal limiting membrane flap technique in patients with macular hole retinal detachment in high myopia. Retin Cases Brief Rep. 2016; 10:140–144.
18. Yoshikawa M, Murakami T, Nishijima K, et al. Macular migration toward the optic disc after inner limiting membrane peeling for diabetic macular edema. Invest Ophthalmol Vis Sci. 2013; 54:629–635.
19. Rodrigues IA, Lee EJ, Williamson TH. Measurement of retinal displacement and metamorphopsia after epiretinal membrane or macular hole surgery. Retina. 2016; 36:695–702.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download