Abstract
Purpose
To report clinical features of patients with retinal and choroidal diseases presenting with acute visual disturbance during pregnancy.
Methods
In this retrospective case series, patients who developed acute visual loss during pregnancy (including puerperium) and visited a tertiary hospital from July 2007 to June 2015, were recruited by searching electronic medical records. Patients were categorized according to the cause of visual loss. Clinical features and required diagnostic modalities were analyzed in the retinal and choroidal disease group.
Results
Acute visual loss occurred in 147 patients; 49 (38.9%) were classified into the retinal and choroidal group. The diagnoses included central serous chorioretinopathy (22.4%), hypertensive retinopathy with or without pre-eclampsia (22.4%), retinal tear with or without retinal detachment (18.4%), diabetic retinopathy progression (10.2%), Vogt-Koyanagi-Harada disease (4.1%), retinal artery occlusion (4.1%), multiple evanescent white dot syndrome (4.1%), and others (14.3%). Visual symptoms first appeared at gestational age 25.9 ± 10.3 weeks. The initial best-corrected visual acuity (BCVA) was 0.27 ± 0.39 logarithm of the minimum angle of resolution (logMAR); the final BCVA after delivery improved to 0.13 ± 0.35 logMAR. Serious visual deterioration (BCVA worth than 20 / 200) developed in two patients. Differential diagnoses were established with characteristic fundus and spectral-domain optical coherence tomography findings in all cases.
Conclusions
In pregnant women with acute visual loss, retinal and choroidal diseases are common and could be vision threatening. Physicians should be aware of pregnancy-associated retinal and choroidal diseases and their clinical features. The differential diagnosis can be established with non-invasive techniques.
Women are more prone to various diseases, including retinal and choroidal diseases, during pregnancy. Retinal and choroidal diseases may occur spontaneously in pregnancy, or pre-existing conditions may worsen or change during pregnancy [1]. Retinal and choroidal diseases include conditions such as central serous chorioretinopathy, hypertensive retinopathy (HTR), and rhegmatogenous retinal detachment, whereas pre-existing conditions include diabetes mellitus, Vogt-Koyanagi-Harada (VKH) disease, and white dot syndrome.
During pregnancy, cardiac output increases as the product of stroke volume and heart rate increase over the course of the pregnancy [2], and many angiopoietic factors increase with hormonal fluctuations, such as changes in progesterone [3]. The combination of these factors plays a role in the initiation or aggravation of retinal and choroidal diseases.
For pregnancy-associated retinal and choroidal diseases, there is a paucity of data regarding the incidence of diseases and evidence on which to base the diagnosis and treatment strategies. Further, pregnant women often refuse to undergo invasive diagnostic tests such as fundus fluorescein angiography (FA), which makes the diagnosis more difficult. Therefore, recognizing the retinal and choroidal diseases that occur during pregnancy and knowing how to diagnose them in the setting of pregnancy, without invasive tests, are important.
Herein, we analyzed the clinical features of pregnancy-associated retinal and choroidal diseases by investigating a series of patients who presented with acute visual disturbance during or after pregnancy.
This study was approved by the institutional review board of Seoul National University Bundang Hospital (SNUBH). The electronic medical records of all female patients of childbearing age who visited SNUBH from July 2007 to June 2015 were screened. The keywords “delivery” and “pregnancy” was used for the search. The inclusion criteria were female patients who developed acute visual loss during pregnancy or within 8 weeks postpartum. All diagnoses were established by ophthalmologists at SNUBH (Fig. 1).
The collected data were patient age, coexisting ocular and/or systemic diseases, initial and final best-corrected visual acuity (BCVA), and intraocular pressure as determined by noncontact tonometry. Information regarding parity, last menstrual period, delivery date, mode of delivery, and obstetric complications such as pre-eclampsia were also gathered. For cases in which the date of delivery was not clarified, gestational age (GA) 40 weeks was assumed to be the delivery date.
Ophthalmologic examinations including fundus photography, FA, and optical coherence tomography (OCT; Spectralis OCT, Heidelberg Engineering, Heidelberg, Germany) were performed. Structural changes in the choroidal layer were examined using the enhanced depth image (EDI) mode of OCT. FA was performed for patients who provided informed consent.
Results are presented as mean ± standard deviation for continuous variables and as percentage for categorical variables. The Mann-Whitney U-test was used to investigate the relationship between initial symptom onset (GA) and diagnosis.
A total of 147 of 571 pregnant women reported acute visual loss during pregnancy and puerperium, and 126 of these patients were diagnosed with ocular diseases. These patients were classified into the following six disease groups: Retinal and choroidal disease (49 cases), external eye disease (58 cases), orbit and eyelid disease (eight cases), anterior uveitis (seven cases), strabismus (two cases), and glaucoma (two cases) (Fig. 1).
We analyzed the 49 patients (66 diseased eyes) in the retinal and choroidal disease group. The mean age ± standard deviation of the retinal and choroid disease group was 33.1 ± 4.2 years (range, 21 to 41 years), and the mean follow-up period was 8.6 ± 14.7 months. The clinical features and non-invasive examinations, including fundus photography and spectral domain-OCT with EDI mode findings, were adequate for establishing detailed diagnoses in all cases. FA was performed on six patients who provided informed consent during pregnancy, but this did not affect the diagnosis or treatment plan. The retinal diagnoses included central serous chorioretinopathy (CSC) (n = 11, 22.4%), HTR with or without pre-eclampsia (n = 11, 22.4%), retinal tear with or without retinal detachment (n = 9, 18.4%), diabetic retinopathy (DR) progression (n = 5, 10.2%), VKH disease (n = 2, 4.1%), multiple evanescent white dot syndrome (n = 2, 4.1%), retinal artery occlusion (n = 2, 4.1%), punctate inner choroidopathy with choroidal neovascularization (n = 1, 2.0%), activation of previous tuberculosis granuloma (n = 1, 2.0%), acute zonal occult outer retinopathy (n = 1, 2.0%), central retinal vein occlusion (n = 1, 2.0%), idiopathic choroidal neovascular membrane with choroiditis (n = 1, 2.0%), macular subretinal hemorrhage (n = 1, 2.0%), and retinoschisis (n = 1, 2.0%) (Table 1). The clinical characteristics of each disease group are described in Table 2, and representative images are shown in Fig. 2A, 2B, 3, 4A, 4B, 5.
The mean GA at symptom onset was 25.9 ± 10.3 weeks. The distribution of initial symptom onset is shown in Fig. 6. During the third trimester (from GA 27 to 40 weeks), 81.8 % cases of HTR, 54.5 % of CSC, and 50.0% of VKH occurred, whereas DR progression (16.7%), retinal tear with or without retinal detachment (11.1%), and retinal artery occlusion (none) showed a much lower incidence in the third trimester. Statistically, the symptom onset of the HTR group was different from those of the other disease groups (GA, 32.8 ± 3.9 weeks; p = 0.002; Mann-Whitney U-test), whereas significant differences were not observed among the other groups.
The mean BCVA ± standard deviation of the retinal and choroidal disease group was 0.27 ± 0.39 logarithm of the minimum angle of resolution (logMAR) at the initial exam and improved to 0.13 ± 0.35 logMAR at the last follow-up exam. The visual prognosis was excellent in most of the cases (Table 2). Serious visual deterioration (BCVA worse than 20 / 200) developed in two patients (4.1%) in whom central retinal artery occlusion and acute zonal occult outer retinopathy occurred, respectively. The visual acuities improved as the subretinal fluid was resolved in CSC and HTR cases, and total disappearance of subretinal fluid was observed only after delivery in all CSC and HTR cases.
Various treatment strategies were applied to the patients: medical treatment (n = 5, 10.2%), surgical treatment (n = 9, 18.4%), prophylactic laser treatment (n = 4, 8.2%), and intravitreal bevacizumab injection (n = 2, 4.1%). Preterm delivery with labor induction or caesarian section was performed in 11 patients (22.4%). The others were observed for spontaneous improvement (n = 18, 36.7%). In the surgically treated group, nine patients (18.4%) underwent ophthalmic surgeries, which included pars plana vitrectomy for three patients (one for vitreous hemorrhage, one for tractional retinal detachment, and one for rhegmatogenous retinal detachment) and scleral buckling for six patients (all for rhegmatogenous retinal detachment). In the medically treated group, four patients (8.2%) were treated with oral prednisolone (two for VKH disease, one for central retinal vein occlusion with macular edema, and one for multiple evanescent white dot syndrome), and one patient (2.0%) started systemic anticoagulant treatment after being diagnosed with acute zonal occult outer retinopathy. In the laser-treated group, three patients with DR (6.1%) were treated with prophylactic laser panretinal photocoagulation, and one patient (2.0%) underwent barrier laser treatment for retinal tear without retinal detachment. Two patients were treated with intravitreal bevacizumab (one for diabetic macular edema and one for choroidal neovascularization).
According to our results, 49 of 147 (33.3%) patients with acute visual disturbance during pregnancy and puerperium were diagnosed as having retinal and choroidal diseases. Among them, 31 patients required treatment. These findings indicate that ophthalmologists should not overlook suspicion of retinal diseases.
CSC is a well-known disease that arises during pregnancy due to hormonal fluctuations, such as changes in progesterone level. HTR is another typical disease during pregnancy, and this condition is induced by severe vascular spasm from pre-eclampsia or eclampsia [4]. In our case series, CSC (n = 11) and HTR (n = 11) occurred only after GA 19 weeks (Fig. 6). When a pregnant patient reports acute visual disturbance in the second half of pregnancy, CSC with unilateral manifestation and HTR with bilateral manifestation should be considered. In all CSC and HTR cases, fetal delivery was followed by spontaneous resolution of subretinal fluid. Although we can confirm that prompt delivery can be a good solution for these cases, it is not regarded as an emergency requirement because the overall visual outcome is generally excellent in these patients. DR is known to progress transiently through pregnancy and can manifest as sudden-onset vitreous hemorrhage or papillitis. Our search revealed five cases of rapid progression of DR, and two of these patients underwent vitrectomy. Conversely, VKH disease is predicted to improve during pregnancy because of steroid production and immune depression, but flare-ups during pregnancy are also reported [56]. We reported two cases of sudden manifestation of VKH disease, which were controlled with systemic corticosteroid treatments. Differential diagnosis with CSC can be achieved by characteristic choroidal appearance (darkening and thickening) on EDI-OCT images. Retinal arterial occlusion is reported in pregnant women in association with migraines, Protein S deficiency, elevation of factor VIII level, and primary antiphospholipid antibody syndrome [78]. Routine blood analysis did not reveal any underlying causes of retinal arterial occlusion in the two cases in our series. The development or recurrence of choroidal neovascularization related to punctate inner choroidopathy has been reported during pregnancy, but a definite causal relationship has not been established [9]. Purtscher retinopathy, which is caused by multiple emboli, has also been reported in pregnancy, but we did not have any such cases in our series [10].
Pregnant women sometimes refuse to undergo diagnostic processes such as FA. Therefore, we focused on the role of spectral domain-OCT with EDI mode as a powerful and noninvasive diagnostic tool for evaluating retinal diseases in pregnant patients. EDI-OCT does not show vascular dynamics, but its high-resolution and enhanced-depth imaging made it possible to diagnose conditions without FA in all cases, especially for CSC and VKH. Further, we reviewed the potential effects of each diagnostic process on fetomaternal health (Fig. 7). Regarding treatment, most retinal diseases regress spontaneously after delivery, but when the condition rapidly deteriorates before approaching full-term, a treatment decision should be made by considering the pros and cons of each treatment modality. Subsequent labor induction or caesarian section can be considered in cases of pre-eclampsia-related retinopathy or VKH syndrome, while consulting an obstetric professional. In cases of DR progression, the condition progresses rapidly to vitreous hemorrhage in a few months, so laser photocoagulation should be performed promptly at the time of diagnosis.
The limitations of this study are the retrospective design and the small number of patients. Further, with the exception of DR cases, the pre-conception ophthalmologic state was not documented. Finally, 14 patients did not revisit the ophthalmologic clinic after delivery because the symptom disappeared.
In conclusion, when a pregnant woman visits an ophthalmologic clinic for acute visual loss, the doctor should evaluate the patient with suspicion of retinal and choroid disease. In most cases, the differential diagnosis can be obtained using non-invasive techniques including spectral-domain OCT. We believe that physicians will find our detailed description of the clinical features and visual outcomes useful.
Figures and Tables
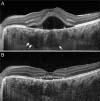 | Fig. 2Optical coherence tomography images in enhanced depth image mode of a patient who was diagnosed with central serous chorioretinopathy at gestational age 25 weeks. (A) The image obtained at gestational age 25 weeks. The dilated large choroidal vessels (arrows), which are characteristic of central serous chorioretinopathy, are visible. (B) The image obtained at 2 weeks postpartum. |
 | Fig. 3Sequential images of a patient who was diagnosed with and underwent treatment for high-risk proliferative diabetic retinopathy during pregnancy (the sequential images were obtained at gestational age (GA) 22 weeks, GA 29 weeks, 5 weeks postpartum, and 10 weeks postpartum). This patient underwent panretinal photocoagulation at GA 22 weeks and received intravitreal injection of bevacizumab at GA 29 weeks. Wide-angle fundus photographs show rapid progression of tractional membrane and the development of preretinal hemorrhage, and the optical coherence tomography images show aggravation and relief of diabetic macular edema. The patient underwent pars plana vitrectomy for tractional retinal detachment 7 months after delivery. |
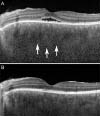 | Fig. 4Images of a patient diagnosed with incomplete Vogt-Koyanagi-Harada during pregnancy. (A) Enhanced depth image optical coherence tomography image of the left eye at gestational age 20 weeks revealing serous retinal detachment with subretinal exudation and choroidal thickening (arrows). (B) Enhanced depth image optical coherence tomography image at gestational age 22 weeks revealing that the subretinal fluid resolved after administration of systemic corticosteroid. |
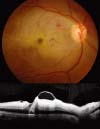 | Fig. 5Fundus photograph and optical coherence tomography image of patient 14, who was diagnosed with central retinal artery occlusion at gestational age 18 weeks. The images were obtained 1 week after the onset of the event, and the acute ischemic changes of the inner retina are apparent. |
 | Fig. 6Initial symptom onset in each disease category. HTR = hypertensive retinopathy; CSC = central serous chorioretinopathy; DR = diabetic retinopathy; RT = retinal tear with or without retinal detachment; RAO = retinal artery occlusion. Symptom onset was significantly different in the HTR group compared with that in the other disease groups (p = 0.002 by the Mann-Whitney U-test), but no differences were observed with the other groups. |
 | Fig. 7 |
Table 2
Clinical manifestations of retinal and choroidal diseases according to diagnosis

Values are presented as mean ± standard deviation unless otherwise indicated.
BCVA = best-corrected visual acuity; logMAR = logarithm of the minimum angle of resolution; RAO = retinal artery occlusion; NLP = no light perception; GA = gestational age; OCT = optical coherence tomography; VEGF = vascular endothelial growth factor.
Acknowledgements
This study was supported by the National Research Foundation of Korea grant 2016R1D1A1B03934724, funded
by the Korean government (Ministry of Science, ICT and Future Planning [MSIP]).
References
1. Errera MH, Kohly RP, da Cruz L. Pregnancy-associated retinal diseases and their management. Surv Ophthalmol. 2013; 58:127–142.
2. Thornburg KL, Jacobson SL, Giraud GD, Morton MJ. Hemodynamic changes in pregnancy. Semin Perinatol. 2000; 24:11–14.
3. Schocket LS, Grunwald JE, Tsang AF, DuPont J. The effect of pregnancy on retinal hemodynamics in diabetic versus nondiabetic mothers. Am J Ophthalmol. 1999; 128:477–484.
4. Saito Y, Tano Y. Retinal pigment epithelial lesions associated with choroidal ischemia in preeclampsia. Retina. 1998; 18:103–108.
5. Doi M, Matsubara H, Uji Y. Vogt-Koyanagi-Harada syndrome in a pregnant patient treated with high-dose systemic corticosteroids. Acta Ophthalmol Scand. 2000; 78:93–96.
6. Rabiah PK, Vitale AT. Noninfectious uveitis and pregnancy. Am J Ophthalmol. 2003; 136:91–98.
7. Acheson JF, Gregson RM, Merry P, Schulenburg WE. Vaso-occlusive retinopathy in the primary anti-phospholipid antibody syndrome. Eye (Lond). 1991; 5(Pt 1):48–55.
8. Greven CM, Weaver RG, Owen J, Slusher MM. Protein S deficiency and bilateral branch retinal artery occlusion. Ophthalmology. 1991; 98:33–34.
9. Sim DA, Sheth HG, Kaines A, Tufail A. Punctate inner choroidopathy-associated choroidal neovascular membranes during pregnancy. Eye (Lond). 2008; 22:725–727.
10. Grant AD, Chung SM. The eye in pregnancy: ophthalmologic and neuro-ophthalmologic changes. Clin Obstet Gynecol. 2013; 56:397–412.
11. Mackensen F, Paulus WE, Max R, Ness T. Ocular changes during pregnancy. Dtsch Arztebl Int. 2014; 111:567–575.
12. American Academy of Pediatrics Committee on Drugs. Transfer of drugs and other chemicals into human milk. Pediatrics. 2001; 108:776–789.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download