Abstract
Purpose
To evaluate the effectiveness of a cycloplegic regimen using 0.5% tropicamide and 0.5% phenylephrine (Tropherine, Hanmi Pharm), in addition to 1% cyclopentolate, in hyperopic children.
Methods
The medical records of hyperopic patients below the age of 14 years who had undergone cycloplegic retinoscopy were retrospectively reviewed. Cycloplegic refractions were performed using one of two cycloplegic regimens. Regimen 1 was a Tropherine-added regimen comprising the administration of one drop of 1% cyclopentolate followed by two to three drops of Tropherine added at 15-minute intervals. Regimen 2 was a cyclopentolate-only regimen comprising the administration of three to four drops of 1% cyclopentolate at 15-minute intervals. The mean difference between noncycloplegic and cycloplegic refraction was compared between the two regimens.
Results
A total of 308 eyes of 308 hyperopic children were included. The mean difference (±standard deviation) in the spherical equivalent (SE) between cycloplegic and noncycloplegic refraction was significantly larger in regimen 2 than in regimen 1, with values of +1.70 ± 1.03 diopters (D) and +1.25 ± 0.89 D, respectively (p=0.001). The SE change after cycloplegia was significantly different between the two regimens only in patients aged 5 years or younger (p=0.001), particularly in those with high hyperopia with an SE ≥5 D (p=0.005) or fully accommodative esotropia (p=0.009). There was no significant difference between the two regimens in patients older than 5 years, regardless of the presence of high hyperopia or fully accommodative esotropia.
Conclusions
The Tropherine-added regimen exerted a weaker cycloplegic effect than the cyclopentolate-only regimen, particularly in children under the age of 5 years with high hyperopia or fully accommodative esotropia. However, the difference in refraction between the two regimens was small. A Tropherine-added regimen can be effective in hyperopic children, with less associated discomfort than the instillation of cyclopentolate.
Cycloplegic refraction is a procedure that allows estimation of a true refractive error by inhibiting accommodation. For fine correction of refractive errors, cycloplegia is necessary, particularly in young children and patients with fully accommodative esotropia or high hyperopia requiring greater accommodative efforts [12].
Atropine sulfate, cyclopentolate hydrochloride, and tropicamide are widely utilized as cycloplegic agents. Although several studies have demonstrated that atropine inhibits accommodation more effectively than cyclopentolate or tropicamide [345], the extremely long duration of action (10 to 15 days) and significant toxicity of atropine with potential side effects of tachycardia, tremor, and delirium make clinicians reluctant to administer atropine for cycloplegic refraction in younger children. Synthetic antimuscarinic agents, such as tropicamide and cyclopentolate, have a shorter duration of action and are more convenient to administer than atropine as alternative cycloplegic agents in clinical settings [2]. Cyclopentolate provides cycloplegia for 12 to 24 hours, while tropicamide is expected to provide cycloplegia for 4 to 10 hours. Findings from a previous survey reveal that some patients prefer tropicamide due to a more rapid return of near vision and a lesser stinging sensation relative to cyclopentolate [6]. Tropicamide requires a shorter duration to obtain maximum cycloplegia, and rarely induces systemic side effects [789]. Early studies [1011] report that the cycloplegic effect of tropicamide is much less than that of atropine or cyclopentolate, and that it is insufficient for cycloplegic refraction in children. In contrast, more recent studies have suggested that cyclopentolate and tropicamide may be equally efficacious for refractive measurements [6121314]. Hyperopic children generally have greater accommodative efforts in comparison to myopic children with relatively lower accommodation requirements [1516]. Therefore, stronger cycloplegic agents are considered necessary for cycloplegic refraction in hyperopic children. Several studies have compared the efficacy of these cycloplegic agents. There is no clear consensus on an optimum cycloplegic agent, however, particularly for hyperopic children.
Recently, mixed eye drops containing 0.5% tropicamide and 0.5% phenylephrine packaged in individual single-use bottles have become commercially available. Instillation of these single-use eye drops for cycloplegic refraction in clinical settings is considerably more convenient and hygienic. The purpose of this study is to evaluate the effectiveness of a cycloplegic regimen using 0.5% tropicamide and 0.5% phenylephrine (Tropherine; Hanmi Pharm, Seoul, Korea), in addition to 1% cyclopentolate, in hyperopic children.
The medical records of patients under the age of 14 years who underwent cycloplegic retinoscopy between January 1 and December 31, 2013, at the Strabismus Center in Kim's Eye Hospital were retrospectively reviewed. Patients with hyperopia of more than +1.00 diopters (D) in the spherical equivalent (SE) (i.e., the sphere plus half of the cylinder) in at least one eye measured by cycloplegic refraction were included. If both eyes were hyperopic with more than +1.00 D in the SE, then only the eye with the larger SE was included in the analysis.
Patients with amblyopia were excluded from the study if they met the criteria of pediatric eye evaluation screening guidelines (i.e., best-corrected visual acuity lower than two lines or more than normal visual acuity for the age of each patient) or if they had any organic eye diseases, including congenital cataract and aphakia.
All children underwent routine ophthalmic evaluations, including slit-lamp examination, cover/uncover tests, prism and alternating cover tests, and fundus photography. Cycloplegic refractions were performed randomly using one of two cycloplegic regimens. Regimen 1 was a Tropherine-added regimen comprising the administration of one drop of 1% cyclopentolate followed by two to three drops of Tropherine added at 15-minute intervals. Regimen 2 comprised a cyclopentolate-only regimen with the administration of three to four drops of 1% cyclopentolate at 15-minute intervals. Cycloplegic retinoscopy was performed between 30 and 60 minutes following the first instillation of cycloplegics, when the pupillary light reflex was eliminated. The ophthalmic technicians were not informed as to which of the cycloplegic agents were applied. Strabismus was categorized as either exotropia, fully accommodative esotropia (defined as esotropia that had been eliminated or reduced to less than 10 prism diopters [PD] by full correction of hyperopia), partially accommodative esotropia (defined as reduced esotropia remaining at 10 PD or greater than 10 PD by full correction of hyperopia), non-accommodative esotropia, other strabismus (mainly hypertropia or dissociated vertical deviation), or no clinically significant strabismus (all heterophoria and heterotropia less than 10 PD). High hyperopia was defined as ≥+5 D in SE by cycloplegic refraction.
The mean difference between noncycloplegic and cycloplegic refraction was compared between the two regimens using the sphere component, cylinder component, and SE. The effects of age, severity of hyperopia, and/or strabismus on refraction differences between the two regimens were evaluated. Numeric values were presented as mean ± standard deviation (SD). Statistical analysis was performed using SPSS ver. 21.0 (IBM Corp., Armonk, NY, USA), and p-values less than 0.05 were considered statistically significant. Student's t-test and chi-square tests were used to compare basic patient characteristics and changes in refraction (spherical and cylinder components, and SE) after instillation of the two different regimens. Two-way analysis of variance was used to compare the changes in refraction between the groups stratified by age, degree of hyperopia, and strabismus. Post-hoc analysis was performed using a t-test with the Bonferroni correction.
A total of 308 children (132 males and 176 females) with a mean age of 3.99 years (SD, 2.30 years; range, 0 to 13 years) were included in this analysis. The SE of manifest refraction was distributed with a mean of +3.95 D (SD, 2.17 D; range, +0.25 to +8.63 D) in this study population. Of the 308 children, 223 received regimen 1 and 85 children received regimen 2 for cycloplegia. There were no significant differences in age, gender, and pre-cycloplegic refraction between the two groups (Table 1).
Changes in refraction after cycloplegia are described in Table 2. More positive values of the SE and sphere components were produced by cycloplegic refraction in comparison to noncycloplegic refraction. The mean changes in SE and sphere components between cycloplegic and noncycloplegic refraction were significantly different, with larger values in regimen 2 than in regimen 1 (p=0.001 for both variables). A minimal difference was found in the cylinder component between regimens after cycloplegia (Table 2).
Children were divided into two groups according to their age at examination (5 years or younger and older than 5 years) and changes in refraction after cycloplegia were compared between the two regimens in both groups (Table 3). There was a statistically significant difference in SE changes after cycloplegia between the two regimens only in the younger age group. Mean changes in regimens 1 and 2 were +1.29 ± 0.88 D and +1.80 ± 1.10 D, respectively (p=0.001) (Table 3). In addition, the mean sphere changes were significantly different only in the younger group, with larger values in regimen 2 in comparison to regimen 1. Mean cylinder changes were not significantly different between the two regimens (Table 3). The effects of the degree of hyperopia (Table 4) or presence of fully accommodative esotropia (Table 5) by age group were also evaluated by stratification. In the group of patients aged 5 years or younger, patients who had high hyperopia with an SE ≥5 D or fully accommodative esotropia revealed larger differences in SE and sphere component changes with regimen 2 than with regimen 1 (p=0.005 and p=0.001 for high hyperopia, respectively and p=0.009 and p=0.006 for fully accommodative esotropia, respectively). Mean cylinder changes were not significantly different between the groups.
This study compares the results of cycloplegic refraction using two different regimens involving Tropherine and cyclopentolate, with a particular focus on hyperopic children. In the total study group, the mean changes in sphere component and SE values between cycloplegic and noncycloplegic refraction were significantly different, with larger values in the cyclopentolate-only regimen group than in the Tropherine-added regimen group. This suggests that the cyclopentolate-only regimen had slightly greater cycloplegic effects and revealed more latent hyperopia than the Tropherine-added regimen. Recently, several studies that compare cyclopentolate to tropicamide in terms of refraction results have found that cyclopentolate has similar or slightly greater cycloplegic effects than tropicamide, and that the differences between the two agents are small [561012131718]. Furthermore, the work of Hofmeister et al. [6] reports that there is no statistically significant difference between tropicamide and cyclopentolate cycloplegic refractions, but that cyclopentolate is more effective than tropicamide at reducing accommodative amplitude in adults. The simple comparison of these findings with our results is not reasonable because of the different study populations. Our study targets hyperopic children, whereas previous studies target myopic or adult patients. These differences in study pools may explain the different cycloplegic effects of cycloplegic agents.
There is no statistically significant difference in cylinder component values after cycloplegia between the two regimens in the present study. Cycloplegia sometimes induces errors in the amount and axis of astigmatism by tightening zonules and tilting lenses in the eyes of patients [19]. Errors in our results in fact presented as minimal cylindrical change, but are not clinically meaningful.
Our study demonstrates that the cyclopentolate-only regimen had statistically significant superiority in cycloplegia efficacy compared with the Tropherine-added regimen, with a difference of 0.42 D between the mean SE changes of regimens 1 and 2. This difference does not appear to be clinically meaningful in cases without amblyopia or strabismus. However, the group of patients aged 5 years or younger, particularly those with high hyperopia with an SE ≥5 D or fully accommodative esotropia, revealed larger differences with the cyclopentolate-only regimen (with a difference of 0.91 D and 0.64 D between the mean SE changes of regimens 1 and 2, respectively). The work of Scobee [20] compares cycloplegia with 1% cyclopentolate and 1% tropicamide, finding no association between the cycloplegic effects of each cycloplegic agent and age of the study population, amount of hyperopia, or presence of esotropia in patients with a lightly pigmented iris. In contrast, the work of Fan et al. [5], which focuses on children with darkly pigmented irises, finds that a combination of 1% tropicamide and 1% cyclopentolate detects significantly larger amounts of hyperopia in patients aged 5 years or younger in comparison to a combination of 0.5% tropicamide and 0.5% phenylephrine in patients of the same age. Our study also targets children with darkly pigmented irises, revealing results that are consistent with the previous research. Recently, Anderson et al. [21] reported that accommodative amplitude measured by objective methods is relatively stable throughout childhood, and does not begin to rapidly decline until the third decade of life. As a physiological response to excessive hyperopia (usually between +2.00 D and +7.00 D), however, a considerable degree of accommodation is required to focus clearly even on distant targets. Such increased accommodative efforts, as in younger children or in patients with high hyperopia and fully accommodative esotropia, may require a stronger cycloplegic agent to produce a sufficient amount of cycloplegia for the performance of cycloplegic refraction.
Herein, there was no significant difference in refraction changes between the two regimens in the group of children older than 5 years and in younger children without fully accommodative esotropia and hyperopia lower than 5 D. This result is consistent with a previous study that compares a 0.5% tropicamide and 0.5% phenylephrine combination with a 1.0% tropicamide and 1.0% cyclopentolate combination [5]. The work of Fan et al. finds that in children older than 5 years or in children without fully accommodative esotropia, both 0.5% and 1.0% combinations have similar cycloplegic efficacy. These findings suggest that a Tropherine-added regimen is sufficient to perform cycloplegic refraction in non-strabismic, low hyperopic children or in older school-aged children.
Both tropicamide and cyclopentolate are synthetic parasympatholytic agents that have more rapid onset times and shorter durations of cycloplegia than atropine. In children, these short-acting agents are preferred for cycloplegic refraction. However, the stinging sensation of cyclopentolate and possible side effects of central nervous system toxicity with visual and tactile hallucinations, cerebellar dysfunction, drowsiness, and grand mal seizures (among others) causes concern among physicians prescribing the drug to children for cycloplegic refraction [222324]. During our study period, no significant side effects of cyclopentolate, including central nervous system toxicity, were observed. The most common side effect of cyclopentolate was a stinging sensation. There were less complaints of discomfort among patients when Tropherine was instilled in comparison to cyclopentolate.
Based on the findings of the present study, a Tropherine-added regimen for cycloplegic refraction is as effective as a cyclopentolate-only regimen, and is convenient to instill with decreased concerns about severe side effects in hyperopic children above the age of 5 years. In contrast, children aged 5 years or younger, particularly those with high hyperopia or fully accommodative esotropia, show greater hyperopic refraction values with a cyclopentolate-only regimen. Therefore, to reveal true refractive states and avoid under-correction of hyperopia in younger children with fully accommodative esotropia or suspected high hyperopia, administration of a cyclopentolate-only regimen is necessary in spite of the associated discomfort and potential side effects. To prevent discomfort in these children, a drop of topical anesthetic, such as proparacaine eye drops, may be used before administration of cyclopentolate eye drops.
Because of the retrospective nature of this study, some limitations are noted. Three different ophthalmic technicians performed refraction for our subjects. Although all of the technicians were experts in pediatric refraction, interpersonal error may have occurred. Moreover, non-cycloplegic and cycloplegic refraction may be performed differently by different technicians for the same children. For consistent, controlled results, additional prospective research with a blinded single technician may be necessary.
In conclusion, our data shows that greater hyperopic results of cycloplegic refraction were produced by administrating a cyclopentolate-only regimen in younger hyperopic children, particularly in those with fully accommodative esotropia and hyperopia over 5 D. However, the hyperopic difference in children without fully accommodative esotropia or in low-grade hyperopic children older than 5 years was not large enough to be clinically meaningful for most situations regarding the prescription of glasses. Furthermore, a Tropherine-added regimen is effective enough to perform cycloplegic refraction with less of a stinging sensation than cyclopentolate. We recommend taking into account the age and strabismic state of children when selecting a regimen for cycloplegic refraction. A Tropherine-added regimen can be used primarily for hyperopic children older than 5 years, but should be used with caution in children aged 5 years or younger with either high hyperopia or with fully accommodative esotropia in consideration of under-correction of hyperopia with or without esotropia.
Figures and Tables
Table 5
Changes in refraction stratified by age and strabismus type
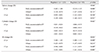
Values are presented as mean ± standard deviation.
D = diopters; ET = esotropia; SE = spherical equivalent.
*p-value by two-way analysis of variance; †p-value <0.0125 by t-test was considered significant with the Bonferroni correction; ‡Children without fully accommodative esotropia were included in the category of “Others.”
References
1. Bujara K, Schulz E, Haase W. Retinoscopy under cycloplegic and non-cycloplegic conditions in children comparison of measurements of three examiners (author's transl). Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1981; 216:339–343.
2. Hiatt RL, Jerkins G. Comparison of atropine and tropicamide in esotropia. Ann Ophthalmol. 1983; 15:341–343.
3. Ingram RM, Barr A. Refraction of 1-year-old children after cycloplegia with 1% cyclopentolate: comparison with findings after atropinisation. Br J Ophthalmol. 1979; 63:348–352.
4. Rosenbaum AL, Bateman JB, Bremer DL, Liu PY. Cycloplegic refraction in esotropic children: cyclopentolate versus atropine. Ophthalmology. 1981; 88:1031–1034.
5. Fan DS, Rao SK, Ng JS, et al. Comparative study on the safety and efficacy of different cycloplegic agents in children with darkly pigmented irides. Clin Exp Ophthalmol. 2004; 32:462–467.
6. Hofmeister EM, Kaupp SE, Schallhorn SC. Comparison of tropicamide and cyclopentolate for cycloplegic refractions in myopic adult refractive surgery patients. J Cataract Refract Surg. 2005; 31:694–700.
7. Gettes BC. Tropicamide, a new cycloplegic mydriatic. Arch Ophthalmol. 1961; 65:632–635.
8. Gettes BC, Belmont O. Tropicamide: comparative cycloplegic effects. Arch Ophthalmol. 1961; 66:336–340.
9. Applebaum M, Jaanus SD. Use of diagnostic pharmaceutical agents and incidence of adverse effects. Am J Optom Physiol Opt. 1983; 60:384–388.
10. Milder B. Tropicamide as a cycloplegic agent. Arch Ophthalmol. 1961; 66:70–72.
11. Lovasik JV. Pharmacokinetics of topically applied cyclopentolate HCl and tropicamide. Am J Optom Physiol Opt. 1986; 63:787–803.
12. Owens H, Garner LF, Yap MK, et al. Age dependence of ocular biometric measurements under cycloplegia with tropicamide and cyclopentolate. Clin Exp Optom. 1998; 81:159–162.
13. Manny RE, Hussein M, Scheiman M, et al. Tropicamide (1%): an effective cycloplegic agent for myopic children. Invest Ophthalmol Vis Sci. 2001; 42:1728–1735.
14. Proskurina OV. Cycloplegic effectiveness of cyclopentolate and tropicamide preparations compared with atropinization. Vestn Oftalmol. 2002; 118:42–45.
15. Rosenfield M, Cohen AS. Repeatability of clinical measurements of the amplitude of accommodation. Ophthalmic Physiol Opt. 1996; 16:247–249.
16. Atchison DA, Capper EJ, McCabe KL. Critical subjective measurement of amplitude of accommodation. Optom Vis Sci. 1994; 71:699–706.
17. Lin LL, Shih YF, Hsiao CH, et al. The cycloplegic effects of cyclopentolate and tropicamide on myopic children. J Ocul Pharmacol Ther. 1998; 14:331–335.
18. Egashira SM, Kish LL, Twelker JD, et al. Comparison of cyclopentolate versus tropicamide cycloplegia in children. Optom Vis Sci. 1993; 70:1019–1026.
19. Duane TD, Jaeger EA, Tasmin W, editors. Duane's foundations of clinical ophthalmology. Philadelphia: JB Lippincott;1994. p. 37.
20. Scobee RG. The nonsurgical treatment of heterotropia. Am J Ophthalmol. 1949; 32:1734–1739.
21. Anderson HA, Hentz G, Glasser A, et al. Minus-lens-stimulated accommodative amplitude decreases sigmoidally with age: a study of objectively measured accommodative amplitudes from age 3. Invest Ophthalmol Vis Sci. 2008; 49:2919–2926.
22. Kennerdell JS, Wucher FP. Cyclopentolate associated with two cases of grand mal seizure. Arch Ophthalmol. 1972; 87:634–635.
23. Khurana AK, Ahluwalia BK, Rajan C, Vohra AK. Acute psychosis associated with topical cyclopentolate hydrochloride. Am J Ophthalmol. 1988; 105:91.
24. Awan KJ. Adverse systemic reactions of topical cyclopentolate hydrochloride. Ann Ophthalmol. 1976; 8:695–698.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download