Abstract
Purpose
To evaluate the long-term outcomes of anti-vascular endothelial growth factor (VEGF) therapy for polypoidal choroidal vasculopathy (PCV) with feeder vessels and to investigate fellow-eye findings.
Methods
This retrospective observational study included 14 eyes with treatment-naïve PCV accompanied by feeder vessels that were treated with anti-VEGF monotherapy. The best-corrected visual acuity (BCVA) at baseline was compared with that at the last follow-up. The fellow-eye indocyanine green angiography findings were also analyzed.
Results
The mean follow-up period was 28.1 ± 19.2 months (range, 12 to 60 months). During the follow-up period, 5.9 ± 2.5 anti-VEGF injections were administered. The logarithm of the minimal angle of resolution (logMAR) BCVAs at the time of diagnosis, at 3 months, and at the last follow-up were 0.81 ± 0.49, 0.55 ± 0.44, and 0.71 ± 0.54, respectively. Although the BCVA at the last follow-up was not different from the baseline value (p=0.809), an improvement of ≥0.2 logMAR BCVA was observed in seven eyes (50.0%). In 11 eyes that underwent bilateral indocyanine green angiography at diagnosis, PCV, branching vascular networks, and late geographic hyperfluorescence were noted in two (18.2%), five (45.4%), and three (27.3%) fellow eyes, respectively. During the follow-up period, the development of polypoidal lesions in the fellow eye was observed in three patients.
Polypoidal choroidal vasculopathy (PCV) is a subtype of choroidal neovascularization (CNV) that is characterized by polypoidal lesions and branching vascular networks (BVNs) [12]. Several groups have attempted to classify PCV on the basis of indocyanine green angiography (ICGA) findings. Yuzawa et al. [3] classified PCV into two subtypes according to the presence of a feeder and a draining vessel [4]. More recently, Tan et al. [5] suggested classifying PCV into three subtypes based on the characteristics of the BVNs and leakage on fluorescein angiography. The classification of PCV is important because treatment outcomes and genetic background may differ depending on the subtype of PCV [56789].
Intravitreal anti-vascular endothelial growth factor (VEGF) is an effective treatment for exudative age-related macular degeneration [10]. Although the efficacy is limited in some cases [11], this therapy has been found to be generally effective in the treatment of PCV [12131415]. A previous study showed that the outcomes of anti-VEGF therapy for PCV with feeder vessels were not different from those for PCV without feeder vessels [16]. However, detailed analyses focused on PCV with feeder vessels have not been performed. In addition, previous studies investigating PCV with feeder vessels primarily focused on the characteristics and treatment outcomes of the involved eye. Findings of the fellow eye in patients with this peculiar type of PCV have not been heavily studied.
The purpose of the present study was to evaluate the long-term outcomes of anti-VEGF therapy for PCV with feeder vessels (which corresponds to PCV type 1 in Yuzawa and colleagues' classification system [34]) and determine the factors associated with visual outcomes. We additionally investigated the findings of the fellow and involved eyes.
This single-institutional, retrospective observational study was approved by the institutional review board and was conducted in accordance with the tenets of the Declaration of Helsinki.
The study included patients diagnosed with treatment-naïve PCV accompanied by feeder vessels. Eyes exhibiting polypoidal lesions on ICGA with or without BVNs were diagnosed with PCV. The presence of feeder vessels was determined on the basis of early-phase ICGA images. The additional inclusion criteria for this study were as follows: (1) follow-up data for at least 12 months, (2) receipt of three monthly intravitreal ranibizumab injections as initial treatment, and (3) receipt of anti-VEGF monotherapy throughout the entire follow-up period. All subjects underwent a comprehensive ophthalmologic examination, including best-corrected visual acuity (BCVA) measurements, 90-diopter lens slit-lamp biomicroscopy, fundus photography, and spectral-domain optical coherence tomography (OCT; Spectral OCT/SLO, OTI Ophthalmic Technologies, Miami, FL, USA). Fluorescein angiography and ICGA were additionally performed using a confocal laser scanning system (HRA-2; Heidelberg Engineering, Dossenheim, Germany). Enhanced-depth imaging OCT [17] (Spectralis, Heidelberg Engineering) was performed at the discretion of each physician. The exclusion criteria for this study were as follows: severe media opacity, a history of photodynamic therapy, a history of vitreoretinal surgery, evidence of end-stage age-related macular degeneration such as central geographic atrophy or disciform scarring, evidence of a macroaneurysm, proliferative diabetic retinopathy, and retinal vascular occlusion. If PCV was bilateral, the eye that was first affected was included.
The visual acuity values were converted to the logarithm of the minimal angle of resolution (logMAR) values for analysis. Central foveal thickness (CFT) was defined as the distance between the internal limiting membrane and Bruch's membrane at the fovea. This measurement was obtained using the calipers provided by an OCT software program. The greatest linear dimension, including the entire BVN and polypoidal lesions, was measured on the ICGA images. The distance between the fovea and the closest polypoidal lesion was also measured. In addition, the incidence of choroidal vascular hyperpermeability, punctate hyperfluorescent spots, and late geographic hyperfluorescence was estimated. Choroidal vascular hyperpermeability was defined as the presence of multifocal hyperfluorescent areas on late-phase ICGA images [18]. A punctate hyperfluorescent spot was defined as a focal hyperfluorescent spot observed on mid-to-late-phase ICGA images [19]. Late geographic hyperfluorescence was defined as a hyperfluorescent lesion with a clearly demarcated geographic margin, which became apparent approximately 10 minutes after the injection of indocyanine green dye [20].
All patients were initially treated with three monthly intravitreal ranibizumab injections. Following initial treatment, the patients were scheduled to visit the hospital once every 1 to 4 months depending on the patient's status. Repeat treatment was performed with intravitreal anti-VEGF (either ranibizumab or bevacizumab) if intraretinal/subretinal fluid remained after the initial injections or if intraretinal/subretinal fluid or retinal/subretinal hemorrhage recurred along with an increase in macular thickness.
The baseline BCVA, BCVA at 3 and 6 months, and BCVA at the final visit were compared. The same comparison was performed with CFT values. The associations of BCVA at the final visit with age, baseline BCVA, baseline CFT, BCVA at 3 months, greatest linear dimension, distance between the fovea and the closest polypoidal lesion, number of anti-VEGF injections, and follow-up duration were also analyzed. Furthermore, the included eyes were divided into two groups according to the morphology of the lesion [4]: an umbrella-like pattern (vessels radiating from a feeder vessel, spreading outward in a radial configuration) and a rake-like pattern (vessels extending from a feeder vessel, resembling the teeth of a rake). The treatment outcomes were evaluated for each group. The incidence of pathological ICGA findings, including PCV, BVNs, and late geographic hyperfluorescence, was evaluated.
Data were recorded as mean ± standard deviation where applicable. All statistical analyses were performed using SPSS ver. 12.0 for Windows (SPSS Inc., Chicago, IL, USA). Differences among various time points were analyzed using repeated-measures analysis of variances, and individual comparisons were performed using Bonferroni's method. Pearson correlation analysis was performed to verify any associations between variables. Multivariable analysis was performed using multiple stepwise linear regressions. Differences between groups were compared using the Mann-Whitney U-test with or without Bonferroni's method. A p-value <0.05 was considered statistically significant.
Fourteen patients (nine men [60.9%] and five women [39.1%]; mean age, 66.7 ± 6.7 years) satisfied all the eligible criteria and were included in the study. Table 1 summarizes the baseline characteristics of the included patients. Choroidal vascular hyperpermeability and punctate hyperf luorescent spots were observed in two (14.3%) and 10 (71.4%) eyes, respectively. Choroidal thickness measurements were performed for 10 eyes with available enhanced-depth imaging OCT findings; the mean thickness was 245.4 ± 52.2 µm.
The mean follow-up period was 28.1 ± 19.2 months (range, 12 to 60 months). All included eyes were initially treated with three monthly intravitreal ranibizumab injections. During the follow-up period, a total of 5.9 ± 2.5 injections, including 4.5 ± 2.1 ranibizumab injections and 1.4 ± 0.8 bevacizumab injections, were administered. Eleven eyes were treated with both ranibizumab and bevacizumab. The remaining four eyes were treated with ranibizumab only.
Fig. 1A-1D shows a representative case of an eye treated with intravitreal anti-VEGF therapy. The BCVAs at baseline, 3 months, and the last follow-up were 0.81 ± 0.49 (Snellen equivalent, 20 / 129), 0.55 ± 0.44 (Snellen equivalent, 20 / 70), and 0.71 ± 0.54 (Snellen equivalent, 20 / 102), respectively (Fig. 2A). At 3 months, complete resolution of fluid in the fovea was observed in 11 eyes (78.6%). At the last follow-up, an improvement of ≥0.2 logMAR BCVA was observed in seven eyes (50.0%), whereas deterioration by ≥0.2 logMAR BCVA was observed in four eyes (28.6%). The remaining three eyes exhibited stable visual acuity (21.4%). BCVA at 3 months was significantly improved compared with that at baseline (p=0.004). However, BCVA at the last follow-up showed no significant differences from the baseline value (p=0.809). CFTs at baseline, 3 months, and the last follow-up were 435.3 ± 153.5 µm, 274.2 ± 128.7 µm, and 296.1 ± 145.0 µm, respectively (Fig. 2B). The CFT measurements obtained at the time of diagnosis were significantly different from those obtained at 3 months and the last follow-up (p<0.001 and p=0.045, respectively).
Table 2 summarizes the results of analyses to determine the factors associated with BCVA at the last follow-up. Univariate analysis revealed baseline BCVA and BCVA at 3 months to be significantly associated with the final BCVA, while multivariate analysis revealed BCVA at 3 months to be the most significantly associated factor.
In total, nine eyes exhibited an umbrella-like pattern and five exhibited a rake-like pattern. The greatest linear dimension, baseline BCVA, and BCVA at the last follow-up were not significantly different between the two groups (p=0.699, p=0.380, and p=0.596, respectively).
Pathologic ICGA findings for the fellow eyes were available for 11 patients (84.6%). Table 3 shows the findings for the fellow eyes of the included patients. Two eyes (18.2%) were diagnosed with bilateral PCV, and five (45.4%) exhibited BVNs only, without polypoidal lesions. Late geographic hyperfluorescence without definite BVNs was observed in three eyes (27.3%). During the follow-up period, the development of polypoidal lesions in the fellow eyes was observed in three of the 12 patients (25.0%) with initially unilateral PCV (Fig. 3A-3F and 4A-4F). Two of these patients exhibited active leakage on fluorescein angiography.
In the present study, the short-term outcomes of anti-VEGF therapy for PCV with feeder vessels were favorable. A significant improvement in visual acuity and a marked decrease in CFT were observed after three monthly ranibizumab injections. However, the visual acuity at a mean of 28.7 months was not significantly different from baseline, even though 50% of the included eyes exhibited an improvement of ≥0.2 logMAR BCVA. The visual acuity measured at 3 months was found to be the most reliable predictor of the final visual outcome.
Yuzawa et al. [3] classified PCV into two types: PCV in the narrow sense (PCV type 2) and polypoidal CNV (PCV type 1) [4]. PCV type 1 was characterized by the presence of feeder vessels and draining venules. PCV type 2 was defined as vascular lesions caused by inner choroidal vessel abnormalities, not by CNV. The size of the lesion was larger in PCV type 1 than in type 2 [4]. In addition, plaque in the late phase ICGA, which is reported to be a characteristic finding of CNV [21], was more frequently noted in PCV type 1 than in PCV type 2 [4]. In OCT images, the highly reflective line representing Bruch's membrane was straight in PCV type 1, whereas the line exhibited irregular thickness and had highly reflective substances adhering to its lower portion in PCV type 2. One of the notable findings in the previous study was that the choroidal thickness was markedly thinner in PCV type 1 than PCV type 2 [4]. A thick choroid is one of the characteristics of PCV that distinguishes it from exudative age-related macular degeneration [2223]. The associated pathological process was suggested to be closely associated with PCV development [24]. For this reason, it is possible that the pathogenesis of the two different subtypes of PCV may differ. Recent genetic studies demonstrated different associations in the genetic variants of the complement factor H gene, age-related maculopathy susceptibility 2 gene, and elastin between the different subtypes of PCV [678]. This result also suggests pathogenic differences between the different subtypes of PCV.
Tan et al. [5] introduced another classification system of the vascular patterns of PCV based on the characteristics of the BVNs and leakage on fluorescein angiography. In their study, cases showing polyps supplied by interconnecting channels were classified as type A PCV, whereas cases showing a distinct BVN were classified as type B (cases without leakage on fluorescein angiography) or type C (cases showing significant late leakage on fluorescein angiography) PCV. The lesion size was smallest for type A and largest for type C. Tan et al. [5] suggested that type A and type B/C PCV may correspond to PCV type 1 and type 2 under the classification system of Yuzawa et al. [3], respectively. Kawamura et al. [4] suggested that efficacy of treatments may differ depending on the type of PCV. In a study by Honda et al. [9], a significant visual improvement after photodynamic therapy was noted in PCV type 2, whereas the visual improvement was limited in PCV type 1. In a study by Tan et al. [5], the treatment outcome was also associated with the subtype of PCV; the best treatment outcome was noted in type A, whereas the worst treatment outcome was noted in type C. These results suggest that classifying PCV is important in establishing a treatment plan and understanding the origin of the disorder.
In a previous study reporting the outcomes of ranibizumab therapy in PCV, the visual prognosis was not associated with the presence of feeder vessels [25]. However, a recent study demonstrated that the treatment outcomes for PCV with a large lesion size were generally poorer than those for PCV with a small lesion size [26]. Considering that PCV with feeder vessels usually exhibits large lesions [45], the treatment outcomes are speculated to be limited. In the present study, the short-term treatment outcomes were favorable. Subretinal or intraretinal fluid in the fovea completely disappeared after the initial three monthly ranibizumab injections in 11 of 14 patients (78.6%). Although the visual acuity at the last follow-up was not significantly different from the baseline value, a definite improvement was observed in 50% of patients. This result suggests that anti-VEGF therapy has a valid effect in some proportion of PCV with feeder vessels.
A large lesion size is associated with poor treatment outcome, regardless of the treatment method [526]. However, the size of the lesion itself was not closely associated with the long-term visual outcomes in our patients. Instead, the visual acuity at 3 months was found to be the most significant predictor of the final visual outcome. In particular, the visual acuity at 3 months, and not the baseline visual acuity, was more closely associated with the final visual outcome, suggesting that the response to anti-VEGF therapy is important for predicting the long-term visual outcome.
A notable finding in the present study was the relatively high incidence of pathological findings in the fellow eye. Two patients were initially diagnosed with bilateral PCV. In these patients, PCV in the fellow eye showed definite feeder vessels. With regard to the remaining nine eyes with available ICGA findings for the fellow eye, 88.9% showed BVNs, late geographic hyperfluorescence, or both. The presence of BVNs or late geographic hyperfluorescence is associated with the development of PCV [27]. In a study by Kim et al. [27], late geographic hyperfluorescence was observed in 23 of 47 eyes (48.9%) with unilateral PCV, six of which also exhibited BVNs. In previous cross-sectional studies, bilateral PCV was observed in approximately 10% to 24% of patients from Asian populations [2829]. In follow-up studies for unilateral PCV, involvement of the fellow eye was observed in 19.1% of patients during a mean follow-up period of 30.3 months [27] and in 11.1% of patients during a 5-year follow-up [30]. The incidence of involvement of the fellow eye in our patients was relatively greater than that reported previously. We postulate that the high incidence of pathological findings in the fellow eye at baseline may have contributed to this finding.
In the present study, the exact symptom duration was either not determined accurately or was longer than 3 months in eight eyes (57.1%). Although the exact reason is uncertain, previous studies have shown that PCV can develop from long-standing Type 1 neovascularization [31]. It is also reported that some patients with Type 1 neovascular tissue are relatively asymptomatic [32]. We postulate that the growth and expansion of feeder vessels may not induce visual disturbances until the polypoidal lesions finally develop. In addition, in the present study, the mean distance between the fovea and polypoidal lesions was approximately one disc diameter. This relatively peripheral location of polyps may also contribute to the delayed recognition of visual disturbances.
Choroidal vascular hyperpermeability and punctate hyperfluorescent spots are frequently observed in PCV patients. The incidence of choroidal vascular hyperpermeability and punctate hyperfluorescent spots was reported to be between 9.8% and 43.5% [33343536] and between 72.4% and 80% [3537], respectively. In the present study, we first evaluated the incidence of these findings in PCVs with feeder vessels. As a result, the incidence of punctate hyperfluorescent spots (71.4%) was comparable with that reported in previous studies, whereas the incidence of choroidal vascular hyperpermeability (14.3%) was relatively lower in our patients. The origin of PCV is not fully understood. The relatively high incidence of choroidal vascular hyperpermeability and punctate hyperfluorescent spots in both patients with PCV and those with central serous chorioretinopathy suggests that these two disorders share a common pathological mechanism [3337]. A previous study showed that the mean choroidal thickness in patients with PCV accompanied by feeder vessels was only 199 µm, whereas that in patients with PCV without feeder vessels was 288 µm [4]. Although the mean choroidal thickness in our patients with available enhanced-depth imaging OCT results (245.4 µm) was relatively greater than the previously reported value for patients with PCV accompanied by feeder vessels, the thickness was also relatively less than that reported for patients with PCV [2223].
Kawamura et al. [4] classified the patterns of PCV with feeder vessels as an umbrella-like pattern and a rake-like pattern. In their study, however, a detailed comparison between the two subtypes was not performed. In the present study, the greatest linear dimension and visual acuity were not different between the two subtypes. Because only a small number of eyes were included, however, further studies with a larger study population are necessary to evaluate the clinical significance of this classification.
The present study had several limitations. First, it was a retrospective study with a small sample size. Second, because enhanced-depth imaging OCT was not routinely performed, the choroidal thickness was measured in a limited proportion of patients. Third, there was no common treatment protocol. Therefore, differences in treatment decisions among clinicians may have influenced the study results.
In summary, anti-VEGF therapy showed short-term benefits for PCV with feeder vessels. Although the long-term efficacy was limited, 50% of the included patients showed a definite improvement in the visual acuity. The visual acuity after three monthly ranibizumab injections was the most reliable predictor of long-term visual outcomes. A relatively high incidence of pathological findings in the fellow eye and bilateral involvement suggest the need for bilateral angiographic and tomographic examinations at the time of diagnosis. Close monitoring of the fellow eye during follow-up is also essential.
Figures and Tables
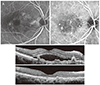 | Fig. 1Fluorescein angiography (A), indocyanine green angiography (B), and optical coherence tomography (C,D) findings at time of diagnosis (A,B,C) and at 57 months after diagnosis (D) in a patient with polypoidal choroidal vasculopathy. The pattern of polypoidal choroidal vasculopathy is classified as an umbrella-like pattern. The patient's visual acuity was 20 / 30 at diagnosis. During the 57-month follow-up period, the patient was treated with 10 intravitreal anti-vascular endothelial growth factor injections, including eight ranibizumab injections and two bevacizumab injections. At 57 months, there was no fluid (D) and an improvement in the visual acuity to 20 / 20. |
 | Fig. 2Changes in best-corrected visual acuity (BCVA, A) and central foveal thickness (CFT, B) in eyes treated with anti-vascular endothelial growth factor, according to the follow-up (FU) period. BCVA significantly improved, while CFT significantly decreased after the initial three monthly ranibizumab injections. However, the values at the last follow-up (mean, 29.6 ± 19.6 months) were not significantly different from those at baseline. |
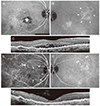 | Fig. 3A representative case of polypoidal choroidal vasculopathy (PCV) with feeder vessels. The pattern of PCV is classified as a rake-like pattern. A the time of diagnosis (A-C), polypoidal lesion (A) and subretinal fluid (C) were noted in the right eye, whereas a branching vascular network and late geographic hyperfluorescence (B, arrowheads) were noted in the left eye. The visual acuity in the right eye was 20 / 60. During the 60-month follow-up period, the right eye was treated with nine intravitreal anti-vascular endothelial growth factor injections, including seven ranibizumab injections and two bevacizumab injections. At 60 months (D-F), there is no fluid in the right eye (F) and an improvement in the visual acuity to 20 / 30. However, marked nasal extension of the PCV lesion was observed (D, arrows). In the left eye, polypoidal lesions developed at the margin of the previously noted late geographic hyperfluorescence (E, double arrowheads). (A,B,D,E) Indocyanine green angiography, (C,F) optical coherence tomography. |
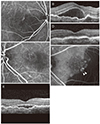 | Fig. 4A representative case of polypoidal choroidal vasculopathy with feeder vessels. At diagnosis (A-D), polypoidal lesions (A) and subretinal fluid (B) were observed in the right eye, whereas the left eye showed type 3 neovascularization without fluid accumulation (C,D). The visual acuity in the right eye was 20 / 100. During the 42-month follow-up period, the right eye was treated with 11 intravitreal anti-vascular endothelial growth factor injections, including eight ranibizumab injections and three bevacizumab injections. At 42 months (E,F), a small amount of subretinal fluid was observed in the right eye (E). The visual acuity in the right eye improved to 20 / 50. In the left eye, however, polypoidal lesions (F, arrowheads) developed. (A,C,F) Indocyanine green angiography, (B,D,E) optical coherence tomography. |
Table 2
Results of analyses to determine factors associated with BCVA at the last follow-up

BCVA = best-corrected visual acuity; CI = confidence interval; VEGF = vascular endothelial growth factor.
*Statistics were analyzed by univariate linear regression analysis; †Statistically significant when tested by stepwise multiple linear regression; ‡Values were measured after three monthly ranibizumab injections.
References
1. Spaide RF, Yannuzzi LA, Slakter JS, et al. Indocyanine green videoangiography of idiopathic polypoidal choroidal vasculopathy. Retina. 1995; 15:100–110.
2. Yannuzzi LA, Sorenson J, Spaide RF, Lipson B. Idiopathic polypoidal choroidal vasculopathy (IPCV). Retina. 1990; 10:1–8.
3. Yuzawa M, Mori R, Kawamura A. The origins of polypoidal choroidal vasculopathy. Br J Ophthalmol. 2005; 89:602–607.
4. Kawamura A, Yuzawa M, Mori R, et al. Indocyanine green angiographic and optical coherence tomographic findings support classification of polypoidal choroidal vasculopathy into two types. Acta Ophthalmol. 2013; 91:e474–e481.
5. Tan CS, Ngo WK, Lim LW, Lim TH. A novel classification of the vascular patterns of polypoidal choroidal vasculopathy and its relation to clinical outcomes. Br J Ophthalmol. 2014; 98:1528–1533.
6. Miki A, Honda S, Kondo N, Negi A. The association of age-related maculopathy susceptibility 2 (ARMS2) and complement factor H (CFH) variants with two angiographic subtypes of polypoidal choroidal vasculopathy. Ophthalmic Genet. 2013; 34:146–150.
7. Tanaka K, Nakayama T, Mori R, et al. Associations of complement factor H (CFH) and age-related maculopathy susceptibility 2 (ARMS2) genotypes with subtypes of polypoidal choroidal vasculopathy. Invest Ophthalmol Vis Sci. 2011; 52:7441–7444.
8. Yanagisawa S, Sakurada Y, Miki A, et al. The association of elastin gene variants with two angiographic subtypes of polypoidal choroidal vasculopathy. PLoS One. 2015; 10:e0120643.
9. Honda S, Miki A, Yanagisawa S, et al. Comparison of the outcomes of photodynamic therapy between two angiographic subtypes of polypoidal choroidal vasculopathy. Ophthalmologica. 2014; 232:92–96.
10. Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006; 355:1419–1431.
11. Cho M, Barbazetto IA, Freund KB. Refractory neovascular age-related macular degeneration secondary to polypoidal choroidal vasculopathy. Am J Ophthalmol. 2009; 148:70–78.e1.
12. Oishi A, Kojima H, Mandai M, et al. Comparison of the effect of ranibizumab and verteporfin for polypoidal choroidal vasculopathy: 12-month LAPTOP study results. Am J Ophthalmol. 2013; 156:644–651.
13. Lim JY, Lee SY, Kim JG, et al. Intravitreal bevacizumab alone versus in combination with photodynamic therapy for the treatment of neovascular maculopathy in patients aged 50 years or older: 1-year results of a prospective clinical study. Acta Ophthalmol. 2012; 90:61–67.
14. Kim JH, Lee DW, Choi SC, et al. Intravitreal anti-vascular endothelial growth factor for newly diagnosed symptomatic polypoidal choroidal vasculopathy with extrafoveal polyps. Korean J Ophthalmol. 2015; 29:404–410.
15. Lee SY, Kim JG, Joe SG, et al. The therapeutic effects of bevacizumab in patients with polypoidal choroidal vasculopathy. Korean J Ophthalmol. 2008; 22:92–99.
16. Hikichi T, Higuchi M, Matsushita T, et al. Factors predictive of outcomes 1 year after 3 monthly ranibizumab injections and as-needed reinjections for polypoidal choroidal vasculopathy in Japanese patients. Retina. 2013; 33:1949–1958.
17. Spaide RF, Koizumi H, Pozzoni MC. Enhanced depth imaging spectral-domain optical coherence tomography. Am J Ophthalmol. 2008; 146:496–500.
18. Guyer DR, Puliafito CA, Mones JM, et al. Digital indocyanine-green angiography in chorioretinal disorders. Ophthalmology. 1992; 99:287–291.
19. Tsujikawa A, Ojima Y, Yamashiro K, et al. Punctate hyperfluorescent spots associated with central serous chorioretinopathy as seen on indocyanine green angiography. Retina. 2010; 30:801–809.
20. Kang SW, Chung SE, Shin WJ, Lee JH. Polypoidal choroidal vasculopathy and late geographic hyperfluorescence on indocyanine green angiography. Br J Ophthalmol. 2009; 93:759–764.
21. Guyer DR, Yannuzzi LA, Slakter JS, et al. Classification of choroidal neovascularization by digital indocyanine green videoangiography. Ophthalmology. 1996; 103:2054–2060.
22. Kim SW, Oh J, Kwon SS, et al. Comparison of choroidal thickness among patients with healthy eyes, early age-related maculopathy, neovascular age-related macular degeneration, central serous chorioretinopathy, and polypoidal choroidal vasculopathy. Retina. 2011; 31:1904–1911.
23. Koizumi H, Yamagishi T, Yamazaki T, et al. Subfoveal choroidal thickness in typical age-related macular degeneration and polypoidal choroidal vasculopathy. Graefes Arch Clin Exp Ophthalmol. 2011; 249:1123–1128.
24. Pang CE, Freund KB. Pachychoroid neovasculopathy. Retina. 2015; 35:1–9.
25. Hikichi T, Higuchi M, Matsushita T, et al. One-year results of three monthly ranibizumab injections and as-needed reinjections for polypoidal choroidal vasculopathy in Japanese patients. Am J Ophthalmol. 2012; 154:117–124.e1.
26. Tsujikawa A, Ojima Y, Yamashiro K, et al. Association of lesion size and visual prognosis to polypoidal choroidal vasculopathy. Am J Ophthalmol. 2011; 151:961–972.e1.
27. Kim YT, Kang SW, Chung SE, et al. Development of polypoidal choroidal vasculopathy in unaffected fellow eyes. Br J Ophthalmol. 2012; 96:1217–1221.
28. Sho K, Takahashi K, Yamada H, et al. Polypoidal choroidal vasculopathy: incidence, demographic features, and clinical characteristics. Arch Ophthalmol. 2003; 121:1392–1396.
29. Byeon SH, Lee SC, Oh HS, et al. Incidence and clinical patterns of polypoidal choroidal vasculopathy in Korean patients. Jpn J Ophthalmol. 2008; 52:57–62.
30. Ueta T, Iriyama A, Francis J, et al. Development of typical age-related macular degeneration and polypoidal choroidal vasculopathy in fellow eyes of Japanese patients with exudative age-related macular degeneration. Am J Ophthalmol. 2008; 146:96–101.
31. Khan S, Engelbert M, Imamura Y, Freund KB. Polypoidal choroidal vasculopathy: simultaneous indocyanine green angiography and eye-tracked spectral domain optical coherence tomography findings. Retina. 2012; 32:1057–1068.
32. Fung AT, Yannuzzi LA, Freund KB. Type 1 (sub-retinal pigment epithelial) neovascularization in central serous chorioretinopathy masquerading as neovascular age-related macular degeneration. Retina. 2012; 32:1829–1837.
33. Sasahara M, Tsujikawa A, Musashi K, et al. Polypoidal choroidal vasculopathy with choroidal vascular hyperpermeability. Am J Ophthalmol. 2006; 142:601–607.
34. Koizumi H, Yamagishi T, Yamazaki T, Kinoshita S. Relationship between clinical characteristics of polypoidal choroidal vasculopathy and choroidal vascular hyperpermeability. Am J Ophthalmol. 2013; 155:305–313.e1.
35. Kim JH, Chang YS, Lee TG, Kim CG. Choroidal vascular hyperpermeability and punctate hyperfluorescent spot in choroidal neovascularization. Invest Ophthalmol Vis Sci. 2015; 56:1909–1915.
36. Hikichi T, Kitamei H, Shioya S. Prognostic factors of 2-year outcomes of ranibizumab therapy for polypoidal choroidal vasculopathy. Br J Ophthalmol. 2015; 99:817–822.
37. Park SJ, Kim BH, Park KH, Woo SJ. Punctate hyperfluorescence spot as a common choroidopathy of central serous chorioretinopathy and polypoidal choroidal vasculopathy. Am J Ophthalmol. 2014; 158:1155–1163.e1.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download