Abstract
Purpose
To report the patient characteristics and treatment outcomes in 12 cases of orbital lymphangioma.
Methods
In this study, orbital lymphangioma was diagnosed based on clinical, radiologic (computed tomography, magnetic resonance imaging), and histologic findings when possible. Patients whose vision was not compromised by orbital lymphangioma, or that did not have increased intraocular pressure (IOP), received oral corticosteroids. Orbital lymphangioma that affected vision or increased IOP was treated by surgery, which included aspiration of blood or partial resection with or without injection of a sclerosant.
Results
Four patients without compromised vision responded well to oral corticosteroids. Eight patients with compromised vision underwent some form of surgery. Bleeding recurred in three patients after aspiration of blood and in two after partial resection and intralesional injection of a sclerosant. Overall, five patients were treated successfully by aspiration of blood, intralesional injection of a sclerosant, and application of continuous negative pressure by appropriate drainage. Partial resection was successful in two patients with organized hematoma.
Conclusions
Orbital lymphangioma that does not compromise vision can be treated medically using oral corticosteroids. Patients with threatened vision or elevated IOP due to acute hemorrhage should be treated by aspiration of blood, intralesional injection of a sclerosant, and application of continuous negative pressure. Partial resection may be effective only in patients with organized hematoma.
Lymphangioma is a benign tumor of the lymphatic system that is characterized by abnormal endothelial-lined channels [1]. Generally found in the head and neck region, these tumors constitute 0.3% to 4% of all orbital tumors and are not considered hamartomas because the orbit does not usually contain lymphatic vessels [23]. Some patients with orbital lymphangioma may develop proptosis, either slowly as the mass invades the orbit or suddenly during hemorrhage of a lesion [3]. In childhood, the diagnosis is often made when proptosis occurs after bleeding as a result of minor trauma or upper respiratory infection, and may even occur spontaneously. The lymphangioma itself or the associated bleeding can restrict ocular motility and cause compressive optic neuropathy because of its mass [4].
Lymphangiomas can also infiltrate diffusely into surrounding vital structures such as the optic nerve. This characteristic, along with the associated hemorrhage, presents many surgical challenges and renders management of orbital lymphangiomas very difficult. Several methods have been used to treat orbital lymphangioma, including systemic corticosteroids, injection of a sclerosant, and surgical excision, but currently, there are no definitive curative treatments [56]. Case reports and studies of orbital lymphangioma are rare in the Korean population. Here we describe the characteristics and treatment outcomes in 12 Korean patients with orbital lymphangioma.
We retrospectively reviewed the medical records of 12 patients that had been diagnosed with orbital lymphangioma between January 2005 and May 2015 at the Severance Hospital, Yonsei University College of Medicine, Seoul, Korea. The diagnosis was based on clinical manifestations, imaging studies, including computed tomography and magnetic resonance imaging (MRI), and histologic findings when possible. Ophthalmic evaluation included measurement of visual acuity, intraocular pressure (IOP), ocular motility, and Hertel exophthalmometry. Patients without elevated IOP and no immediate threat to vision were treated with oral corticosteroids. Patients threatened with loss of vision or severe disfigurement underwent aspiration of blood or partial resection (with or without intralesional injection of a sclerosant and application of continuous negative pressure). The study adhered to the tenets of the Declaration of Helsinki. Ethical approval to conduct the study was obtained from the institutional review board at the Severance Hospital of Yonsei University (No. 4-2015-0342).
The demographic and clinical characteristics of the patients are detailed in Table 1. The mean patient age at presentation was 14.7 years (range, 1 to 76; median, 5.5). Seven patients were male and five were female. Ten patients were diagnosed with orbital lymphangioma in childhood. The mean follow-up period was 22.8 months (range, 2 to 68). All of the patients initially presented with proptosis due to lesional hemorrhage and mass effect of the tumor. The causes of bleeding included blunt ocular trauma (n = 3), upper respiratory infection (n = 2), and a spontaneous event (n = 7). The tumors were classified as superficial (n = 0), deep (n = 6), combined (n = 4), or complex (n = 2) [5].
In patients that could be tested for visual acuity (n = 10), all but one had a visual acuity at presentation above 20 / 50. One patient had a visual acuity of 10 / 200 and two patients could not be tested because of their young age. During treatment, one patient showed worsening of visual acuity to light perception negative, but the other patients did not experience any further impairment of visual acuity. An IOP above 21 mmHg was recorded in four patients. All patients had limited ocular motility and three had compressive optic neuropathy before or during follow-up.
Four patients (cases 6, 8, 11, and 12) were treated with oral corticosteroids only (Fig. 1A and 1B), but eight required some form of surgical intervention. Five of the eight surgically treated patients experienced a recurrence of one or more hemorrhagic episodes. Among these, two patients (cases 4 and 7) underwent a partial resection and received an intralesional injection of a sclerosant, and three patients (cases 1, 5, and 10) underwent aspiration of blood (Fig. 2). In the two patients who underwent partial resection (cases 4 and 7), bleeding recurred from posterior residual lymphangioma tissue. At reoperation, this residual tissue was difficult to approach due to its deep location. Overall, five patients (cases 1, 2, 3, 4, and 7) were treated successfully by aspiration of blood, intralesional injection of a sclerosant (OK-432 in two cases and bleomycin in three cases), and application of continuous negative pressure by appropriate drainage. Two patients (cases 9 and 10) experienced organized hematoma that caused proptosis for several months, but after partial resection, these patients eventually had favorable outcomes (Table 1).
A 4-year-old girl presented with a 1-day history of left-sided proptosis and eyelid swelling (Fig. 3A). She had been treated with OK-432 (Picibanil; Chugai Pharmaceutical, Tokyo, Japan) for a lymphatic malformation on her left forehead, frontal and temporal scalp 3 years previously, and the lesions had shrunk and little remained. Visual acuity in her affected eye was 20 / 30 and the IOP was 20 mmHg. Ocular motility was restricted in all directions and the degree of proptosis was 13 mm on the right and 19 mm on the left. MRI confirmed a diagnosis of lymphangioma, which consisted of multiple diffusely infiltrating cysts with fluid-fluid levels (Fig. 3B and 3C). The following day, the lesion was approached with a left upper eyelid subbrow incision (Fig. 3D). Blood was aspirated, the lymphangioma was partially resected (Fig. 3E), and 0.05 mg of OK-432 diluted in 1 mL of physiologic saline was injected into the deep residual mass. The size of the mass subsequently decreased, which relieved the proptosis (Fig. 3F).
Thirty months later, the patient presented again after developing severe painful proptosis and eyelid swelling, with vision that had decreased to 20 / 200 (Fig. 3G). A grade 2 relative afferent pupillary block was noted in her left eye. MRI showed recurrence of bleeding from the residual lymphangioma tissue (Fig. 3H). Blood was aspirated via the previous incision site and bleomycin (1 IU per mL of saline) was injected intralesionally over 10 minutes. A drain was inserted but did not function well. Bleeding recurred on the following day and the patient's proptosis worsened. The previous incision site was reopened; blood was evacuated with a suction catheter; and the same concentration of bleomycin was injected into the residual mass over 10 minutes. Another drain was inserted and continuous negative pressure was applied, with marked resolution of the proptosis. Oral corticosteroids (10 mg/day) were given for 2 weeks and then tapered. The final visual acuity in the affected eye was light perception negative, most likely due to compression of the optic nerve by intraorbital hemorrhage. The hemorrhage around the optic nerve was evacuated carefully, so it is unlikely that damage to the optic nerve was caused by the surgery itself. The proptosis normalized 2 months after reoperation (Fig. 3I), and no additional hemorrhagic episodes have occurred in 12 months of follow-up observation.
A 1-year-old boy presented with a 1-month history of right proptosis. He had previously been diagnosed with cystic lymphangioma on the right forehead. The forehead lesion had been surgically excised 7 months after birth, and remained small in size. MRI showed non-encapsulated lobulated intraconal and extraconal cystic masses in the right orbit. These were treated by aspiration of 5 mL of blood, partial resection of the mass, and an injection of OK-432 0.05 mg in 5 mL of physiologic saline into the anterior orbitotomy site over 5 minutes. Two days later, the patient's proptosis worsened because of hemorrhage, so 12 mL of blood was aspirated through the previous opening site and drainage material was inserted. Then, 10 mg of prednisolone was started on a tapering schedule, and the proptosis decreased. Four months after surgery, proptosis recurred in the right eye. MRI revealed a hemorrhage behind the previous lesion, so 12.5 mg of oral corticosteroids were administered and the proptosis slowly subsided. Six months later, severe eyelid swelling and proptosis were observed. MRI showed an increase in size of the lymphangioma and hemorrhage in the right orbit. Blood was aspirated from the mass lesion and a sclerosant (bleomycin, 1 IU per mL of saline) was injected into the cyst over 5 minutes. Drainage material was then inserted to create negative pressure inside the lesion. After surgery, oral corticosteroids and sildenafil (3.3 mg/day) were started. The sildenafil was not associated with any adverse reactions and no additional hemorrhagic episodes have occurred during 6 months of follow-up.
A 19-year-old man was referred to our clinic with left-sided proptosis and eyelid swelling. He had been diagnosed with orbital lymphangioma 16 years previously. Visual acuity in the left eye was 20 / 30; IOP was 16 mmHg; motility was restricted; and the degree of proptosis was 20 mm (13 mm on the right). MRI findings were consistent with orbital lymphangioma. At the time of the initial diagnosis, the patient had good visual acuity and IOP in the normal range, so he initially declined treatment. Three years later, severe proptosis (28 mm on exopthalmometry) developed in his left eye following an upper respiratory infection which required intervention, and 5 mL of blood was aspirated and oral corticosteroid therapy (40 mg/day) was started on a tapering schedule. His symptoms seemed to improve for 6 weeks, but then worsened. The degree of left-sided proptosis increased to 35 mm (Fig. 4A and 4B), and MRI showed large diffuse cysts with an organized hematoma in the left orbit (Fig. 4C and 4D). After discussion with a radiologist, the affected orbit was irradiated with 20 Gy; however, this did not improve his proptosis or conjunctival edema. Sildenafil, at a dose of 25 mg/day, was started in an attempt to reduce the lesion size [7]. The mass was then carefully resected via a lateral orbitotomy and a drain was inserted into the remaining lesion. After surgery, the degree of proptosis was reduced to 16 mm and visual acuity was unimpaired, although hypotropia developed, along with ocular motility limitations (Fig. 4E and 4F). During 8 months of follow-up, hemorrhagic episodes have not recurred in this patient.
Orbital lymphangioma is often detected when intralesional hemorrhage induces proptosis, which can occur either suddenly or gradually. Compressive optic neuropathy can develop when the hemorrhage is massive or the tumor itself compresses the optic nerve. Tunc et al. [8] reported that 85% of their patients with orbital lymphangioma had proptosis; 73% had ptosis; and 46% had reduced ocular motility. In our case series, all 12 patients had proptosis and limited ocular motility at presentation. Two patients had compressive optic neuropathy at presentation and one patient had visual acuity that deteriorated to light perception negative, most likely because of prolonged compression of the optic nerve by intraorbital hemorrhage.
Orbital lymphangioma can be classified into four types based on location [59]. The superficial type is characterized by subcutaneous or conjunctival lesions; the deep type shows orbital invasion; the combined type has superficial and deep components; and the complex type involves other head and neck structures. The tumors in our study were classified as deep, combined, and complex in six, four, and two patients, respectively. The mean age of our patients at presentation was 14.7 years, and 10 were diagnosed with orbital lymphangioma in childhood. Because of the frequent early onset, treatment for orbital lymphangioma is directed at preventing amblyopia and preserving visual acuity [5].
On computed tomography, orbital lymphangioma appears as a mildly enhancing cystic mass. MRI usually shows findings similar to those of computed tomography, but has better diagnostic accuracy because of higher resolution, and is safer because of the lack of ionizing radiation [1011]. MRI may also indicate the presence of feeder vessels, in which case a diagnosis of orbital lymphangioma is excluded [121314]. Overall, radiologic analysis alone has a diagnostic specificity of 77% for this type of tumor. However, orbital lymphangioma can sometimes be misdiagnosed as orbital hemangioma, rhabdomyosarcoma, or lymphoma when only imaging studies are used [7]. For this reason, in addition to diagnosing the tumor based on clinical and radiologic findings, we also confirmed the diagnosis histologically when a surgical intervention was performed.
Diffuse infiltration and associated hemorrhage make this type of tumor difficult to remove surgically. Oral corticosteroid therapy alone was effective in four of our patients, which is consistent with previous reports [1415]. Systemic corticosteroids reduce lymphoid hypertrophy and stabilize the abnormal vasculature, leading to resolution of the lymphangioma. Therefore, orbital lymphangiomas do not necessarily require surgical intervention unless there are vision-threatening or severely disfiguring lesions [1516].
Another useful modality for treating lymphangioma is intralesional injection of a sclerosant such as OK-432 or bleomycin [3517]. OK-432 is prepared from a low-virulence Su strain of group A Streptococcus pyogenes of human origin treated with penicillin G. It has been used to treat malignant tumors, pleural effusion, and malignant ascites [1819]. According to Giguere et al. [20], injection of OK-432 decreases the size of head and neck lymphangiomas. This claim is supported by case reports which demonstrated that orbital lymphangiomas are treatable with in tralesional OK-432 [2122]. Bleomycin, first isolated from Streptomyces verticillus in 1966 [23], has a sclerosing effect on vascular endothelial cells and has been used to treat pleural effusion and vascular anomalies [24]. A literature search revealed that there were a few reports on the use of bleomycin injections to treat orbital lymphangioma, with no systemic or ophthalmic complications [425]. In our case series, five patients were successfully treated with intralesional injections of a sclerosant and application of continuous negative pressure. However, hemorrhage recurred in patients who received injection of a sclerosant without application of continuous negative pressure. Thus, it is recommended that this modality be combined with aspiration of blood and application of continuous negative pressure using a correctly positioned drain.
Two patients received oral sildenafil in our study. Sildenafil causes cystic lesions to collapse by decreasing the contractility of vascular smooth muscle [726]. However, we did not confirm this effect in our patients. The most common adverse reactions of sildenafil are pyrexia, gastrointestinal discomfort, cough, sleep disturbance, and migraine, but these were not observed in our cases. In one patient, orbital lymphangioma was fractionally irradiated with 20 Gy, as recommended by Portnow et al. [27], with the goal of reducing the size of the lesion. However, lymphangioma is not a rapidly proliferating lesion, so radiotherapy was ineffective for this purpose.
Five patients in our case series experienced recurrent hemorrhage. Two patients that had undergone partial resection and intralesional injection of a sclerosant during an acute hemorrhagic episode experienced recurrence of hemorrhage from the remaining lymphangioma tissue several months after treatment. The posterior location of the residual mass made it difficult to evacuate blood and inject the sclerosant in these patients. Therefore, these findings indicate that the surgical approach becomes more difficult when bleeding occurs after partial resection. MRI showed that the mass tended to bulge toward the retrobulbar intraconal space, because adhesions that arose after previous surgery blocked any superficial expansion. We hypothesize that a decrease in the available expansion space could increase the risk of compressive optic neuropathy. Three patients who only underwent aspiration of blood also experienced recurrent hemorrhage, but blood could be easily removed through the previous incision site. In comparison, the patients in this study had favorable outcomes when partial resection was applied, as demonstrated by the two cases of orbital lymphangioma that were associated with organized hematoma.
In summary, orbital lymphangioma that is not threatening to vision can be medically treated with oral corticosteroids. We suggest that aspiration of blood, intralesional injection of a sclerosant, and appropriate drainage to maintain continuous negative pressure are only necessary for patients that have threatened vision, as we reported previously [4]. Additionally, in cases of orbital lymphangioma that are associated with organized hematoma, partial resection can be considered to debulk the mass.
This case series describes manifestations and treatment outcomes of orbital lymphangioma in the Korean population. Our report helps to further knowledge on the clinical course, prognosis, and appropriate treatment modalities for patients with orbital lymphangioma.
Figures and Tables
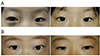 | Fig. 1Patients with orbital lymphangioma that were successfully treated with oral corticosteroids. (A) Case 6, (B) case 8 (left column, pretreatment; right column, posttreatment). |
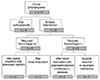 | Fig. 2Treatment modalities of patients with orbital lymphangioma who underwent surgical intervention and experienced recurrent hemorrhage. |
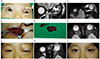 | Fig. 3Four-year-old girl with left orbital lymphangioma. (A) Proptosis at initial visit. (B) Axial T2-weighted magnetic resonance image showing multiple diffusely-infiltrating cysts. (C) Coronal T2-weighted magnetic resonance image showing cysts with fluid-fluid levels. (D) Lymphangioma observed via the anterior orbitotomy site. (E) Partially-resected lymphangioma. (F) Axial T2-weighted magnetic resonance image showing that the cystic mass had been removed from the left orbit. (G) Bleeding recurred 30 months after the first surgical intervention. (H) Axial T2-weighted magnetic resonance image indicating bleeding from deep residual lymphangiomatous tissue. (I) Appearance 6 months after the repeated surgery; bleeding has not recurred, but the patient's visual acuity has changed to no light perception. |
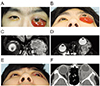 | Fig. 4Nineteen-year-old man with a 16-year history of left orbital lymphangioma. (A) Severe proptosis and conjunctival swelling before resection. (B) Exophthalmometry showing 35-mm protrusion of the left eyeball. (C) Coronal T2-weighted magnetic resonance image showing lymphangioma that occupied most of the left orbit. (D) Axial T2-weighted magnetic resonance image showing a large lymphangioma with organized hematoma anteriorly displacing the left eyeball. (E) Reduced proptosis after partial resection. (F) Computed tomography image after partial resection. |
Acknowledgements
This research was supported by the Bio & Medical Technology Development Program of the NRF funded by the Korean Government, Ministry of Science, ICT and Future Planning (2015M3A9E206703).
References
1. Guinto G, Guinto-Nishimura Y. Orbital lymphangiomas. World Neurosurg. 2014; 81:708–709.
2. Rootman J, Hay E, Graeb D, Miller R. Orbital-adnexal lymphangiomas: a spectrum of hemodynamically isolated vascular hamartomas. Ophthalmology. 1986; 93:1558–1570.
3. Russin JJ, Rangel-Castilla L, Kalani MY, Spetzler RF. Surgical management, outcomes, and recurrence rate of orbital lymphangiomas. J Clin Neurosci. 2015; 22:877–882.
4. Lee KH, Han SH, Yoon JS. Successful treatment of orbital lymphangioma with intralesional bleomycin and application of continuous negative pressure. Korean J Ophthalmol. 2015; 29:70–72.
5. Saha K, Leatherbarrow B. Orbital lymphangiomas: a review of management strategies. Curr Opin Ophthalmol. 2012; 23:433–438.
6. Boulos PR, Harissi-Dagher M, Kavalec C, et al. Intralesional injection of Tisseel fibrin glue for resection of lymphangiomas and other thin-walled orbital cysts. Ophthal Plast Reconstr Surg. 2005; 21:171–176.
7. Gandhi NG, Lin LK, O'Hara M. Sildenafil for pediatric orbital lymphangioma. JAMA Ophthalmol. 2013; 131:1228–1230.
8. Tunc M, Sadri E, Char DH. Orbital lymphangioma: an analysis of 26 patients. Br J Ophthalmol. 1999; 83:76–80.
9. Jastrzebski A, Brownstein S, Manusow J, Rubab S. Isolated conjunctival lymphangioma. Can J Ophthalmol. 2011; 46:369–370.
10. Haik BG, Saint Louis L, Smith ME, et al. Magnetic resonance imaging of orbital lymphangiomas. Am J Ophthalmol. 1987; 103:724–725.
11. Kazim M, Kennerdell JS, Rothfus W, Marquardt M. Orbital lymphangioma: correlation of magnetic resonance images and intraoperative findings. Ophthalmology. 1992; 99:1588–1594.
12. Bisdorff A, Mulliken JB, Carrico J, et al. Intracranial vascular anomalies in patients with periorbital lymphatic and lymphaticovenous malformations. AJNR Am J Neuroradiol. 2007; 28:335–341.
13. Bond JB, Haik BG, Taveras JL, et al. Magnetic resonance imaging of orbital lymphangioma with and without gadolinium contrast enhancement. Ophthalmology. 1992; 99:1318–1324.
14. Graeb DA, Rootman J, Robertson WD, et al. Orbital lymphangiomas: clinical, radiologic, and pathologic characteristics. Radiology. 1990; 175:417–421.
15. Harris GJ, Sakol PJ, Bonavolonta G, De Conciliis C. An analysis of thirty cases of orbital lymphangioma: pathophysiologic considerations and management recommendations. Ophthalmology. 1990; 97:1583–1592.
16. Wilson ME, Parker PL, Chavis RM. Conservative management of childhood orbital lymphangioma. Ophthalmology. 1989; 96:484–489.
17. Wiegand S, Eivazi B, Zimmermann AP, et al. Sclerotherapy of lymphangiomas of the head and neck. Head Neck. 2011; 33:1649–1655.
18. Gochi A, Orita K, Fuchimoto S, et al. The prognostic advantage of preoperative intratumoral injection of OK-432 for gastric cancer patients. Br J Cancer. 2001; 84:443–451.
19. Katano M, Morisaki T. The past, the present and future of the OK-432 therapy for patients with malignant effusions. Anticancer Res. 1998; 18:3917–3925.
20. Giguere CM, Bauman NM, Sato Y, et al. Treatment of lymphangiomas with OK-432 (Picibanil) sclerotherapy: a prospective multi-institutional trial. Arch Otolaryngol Head Neck Surg. 2002; 128:1137–1144.
21. Suzuki Y, Obana A, Gohto Y, et al. Management of orbital lymphangioma using intralesional injection of OK-432. Br J Ophthalmol. 2000; 84:614–617.
22. Yoon JS, Choi JB, Kim SJ, Lee SY. Intralesional injection of OK-432 for vision-threatening orbital lymphangioma. Graefes Arch Clin Exp Ophthalmol. 2007; 245:1031–1035.
23. Saitta P, Krishnamurthy K, Brown LH. Bleomycin in dermatology: a review of intralesional applications. Dermatol Surg. 2008; 34:1299–1313.
24. Muir T, Kirsten M, Fourie P, et al. Intralesional bleomycin injection (IBI) treatment for haemangiomas and congenital vascular malformations. Pediatr Surg Int. 2004; 19:766–773.
25. Gooding C, Meyer D. Intralesional bleomycin: a potential treatment for refractory orbital lymphangiomas. Ophthal Plast Reconstr Surg. 2014; 30:e65–e67.
26. Swetman GL, Berk DR, Vasanawala SS, et al. Sildenafil for severe lymphatic malformations. N Engl J Med. 2012; 366:384–386.
27. Portnow LH, Scott M, Morris CG, et al. Fractionated radiotherapy in the management of benign vascular tumors. Am J Clin Oncol. 2012; 35:557–561.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download