Abstract
Congenital cranial dysinnervation disorders are a group of diseases caused by abnormal development of cranial nerve nuclei or their axonal connections, resulting in aberrant innervation of the ocular and facial musculature. Its diagnosis could be facilitated by the development of high resolution thin-section magnetic resonance imaging. The purpose of this review is to describe the method to visualize cranial nerves III, IV, and VI and to present the imaging findings of congenital cranial dysinnervation disorders including congenital oculomotor nerve palsy, congenital trochlear nerve palsy, Duane retraction syndrome, Möbius syndrome, congenital fibrosis of the extraocular muscles, synergistic divergence, and synergistic convergence.
Congenital cranial dysinnervation disorders (CCDDs) are a group of diseases caused by abnormal development of cranial nerve nuclei or their axonal connections, resulting in aberrant innervation of the ocular and facial musculature [1]. Their diagnosis could be facilitated by the development of high resolution thin-section magnetic resonance imaging (MRI). In this review, we describe a method to visualize cranial nerves (CNs) III, IV, and VI and present imaging findings of CCDDs including congenital CN III palsy, congenital CN IV palsy, Duane retraction syndrome, Möbius syndrome, congenital fibrosis of the extraocular muscles, synergistic divergence, and synergistic convergence.
Cranial nerve imaging is usually performed using thin-section T2-weighted imaging based on gradient-echo imaging or turbo spin-echo sequence, which shows CNs as dark linear structures in contrast to the high signal intensity of surrounding cerebrospinal fluid [23].
For the imaging of CNs III and VI, thin-section T2-weighted imaging is performed in an axial plane at the level of the brainstem including the lower midbrain, pons, and upper medulla oblongata [2]. Image resolution (voxel size) of approximately 0.5 × 0.5 mm in the x-y plane and 0.7 mm of section thickness is sufficient to identify the nerves because the mean diameters of CN III has been reported to be 1.8 to 1.9 mm in a previous MRI study [4]. Although the size of CN VI has not been quantitatively measured in previous MRI studies, its diameter is approximately 1.0 mm according to our experience. Therefore, both nerves can be evaluated with a single imaging method using a 1.5- or 3-tesla MRI system. CN III emerges from the anterior surface of the lower midbrain and courses in the anterolateral direction toward the cavernous sinus (Fig. 1A). CN VI emerges from the pontomedullary sulcus, coursing in the anterosuperior direction toward Dorello's canal (Fig. 1B). In addition, CN VII and CN VIII also merge from the pontomedullary sulcus lateral to the root exit of CN VI (Fig. 1B); therefore, these nerves can be evaluated together.
CN IV should be imaged with higher spatial resolution than the other CNs because it is very small (mean diameter, 0.54 mm) [5]. Therefore, image resolution (voxel size) of approximately 0.3 × 0.3 mm in the x-y plane and 0.3 mm of section thickness is needed to depict CN IV clearly, which can be achieved only with a 3-tesla MRI system. Imaging should be conducted at the level of the lower midbrain and upper pons, including the inferior margin of the inferior colliculus [56]. The oblique axial imaging plane is recommended to be perpendicular to the long axis of the aqueduct (i.e., imaging plane being approximately parallel to the course of the CN IV). CN IV exits from the inferior margin of the inferior colliculus and courses in the anterolateral direction toward the ipsilateral tentorium in the perimesencephalic cistern (Fig. 2).
MR imaging findings of congenital CN III palsy varied from aplasia, hypoplasia, to normal [789]. Lim et al. [8] reported that only one out of three patients showed bilateral CN III hypoplasia or hypoplasia of involved extraocular muscles, with normal-sized CN III noted in the two other patients. Kim and Hwang [9] also found unilateral CN III hypoplasia in one out of three patients and reported that the degree of atrophy of the superior, inferior, and medial recti increased with increasing age in the three patients (Fig. 3A–3C), even though the patient number was too small to reach any conclusions.
In addition, brain abnormalities including developmental brain anomaly in the basal ganglia, optic nerve hypo-/dysplasia, pituitary gland malformation, hypoplasia of the midbrain and corpus callosum, septo-optic dysplasia (de Morsier syndrome), ventricular dilatation, and absence of the septum pellucidum have been reported [1011121314].
Sato [15] first reported significantly smaller superior oblique muscle in patients with congenital superior oblique palsy. She also found more severe hypertropia in the primary position and loose tendon on traction test under general anesthesia in patients with smaller superior oblique muscle belly on MRI. Demer et al. [16] also reported absent or atrophic superior oblique muscle in superior oblique palsy. Superior oblique hypoplasia has been reported in familial cases [17].
In 2010, Choi et al. [5] first consistently visualized CN IV using high-resolution 3-tesla (3T) MRI. Kim and Hwang [18] first reported absent CN IV in 10 patients with hypoplasia of the superior oblique muscles with congenital CN IV palsy and asserted that congenital CN IV palsy with superior oblique hypoplasia also can be classified as a CCDD based on documentation with MRI (Fig. 4A–4C). Yang et al. [6] demonstrated that 73% of 97 patients with congenital CN IV palsy showed CN IV aplasia and variable degrees of superior oblique hypoplasia. Patients without CN IV showed more frequent head tilt (96% vs. 81%) at an earlier onset before 1 year of age (80% vs. 29%) and a larger degree of hypertropia on ipsilateral head tilt. Patients with CN IV more frequently showed inferior oblique overaction and dissociated vertical deviation.
Lee et al. [19] performed a morphologic study of CN IV and superior oblique muscle in three groups of control and CN IV palsy with and without CN IV and showed a positive correlation between the diameter of CN IV and the superior oblique volume in controls and in the nonparetic sides of congenital CN IV palsy. However, they found no linear correlation between CN IV diameter and superior oblique volume in the paretic side of congenital CN IV palsy patients with CN IV. In another morphologic study, Yang et al. [20] showed significantly smaller superior oblique muscle volume of the paretic side in patients without CN IV. The cutoff value of the superior oblique muscle volume ratio for diagnosing CN IV absence was 0.75 (sensitivity, 98.9%; specificity, 95.0%) in patients with congenital CN IV palsy. These results suggest that the ratio of paretic/normal side superior oblique volume could be very useful for predicting CN IV absence in congenital CN IV palsy.
Some patients with congenital CN IV palsy without CN IV can develop diplopia for the first time in their 60s or 70s. A patient could develop diplopia after a phacoemulsification of cataract under topical anesthesia [21]. In such cases, the diagnosis of congenital CN IV palsy could be challenging, and MRI could be helpful for the correct diagnosis [22]. Rarely, a patient without CN IV presents with transient diplopia and might show no significant superior oblique underaction on versions [23]. More interestingly, a patient showed superior oblique underaction on the contralateral side to CN IV aplasia [24]. Therefore, a 3T MRI system with a suitable protocol to visualize CN IV could sometimes be essential for correct diagnosis.
Okanobu et al. [25] reported the lateral rectus split into superior and inferior portions in the posterior orbit in one (4%) of 26 patients with congenital CN IV palsy.
Duane's retraction syndrome (DRS) is characterized by congenital abduction limitation, palpebral fissure narrowing on adduction caused by retraction of the globe, and upshoots or downshoots on adduction [26]. There are three types of this syndrome: abduction limitation in type 1 DRS, adduction limitation in type 2 DRS, and limited abduction and adduction in type 3 DRS [27]. There have been pathologic reports of type 1 and 3 DRS in which the abducens nuclei and CN VI were hypoplastic or absent from the brainstem, and the lateral rectus muscle was innervated by CN III branches [2829]. Parsa et al. [30] first reported the absence of CN VI in a patient with type 1 DRS using MRI. Kim and Hwang [2] first reported that the presence of CN VI depends on the type of DRS: CN VI is absent in type 1 DRS (Fig. 5A and 5B), present in type 2 DRS, and can be either present or absent in type 3 DRS patients. Later, Demer et al. [7] found that CN VI was small or absent in the affected orbit, and CN III was in continuity with CN VI, suggesting aberrant innervation of the lateral rectus muscle by CN III. Six years later, Kim and Hwang [31] found that CN VI was present in all 13 eyes of 12 patients with type 2 DRS. However, Denis et al. [3233] found a severely hypoplastic orbital CN VI in a patient with type 2 DRS in whom they could not identify the cisternal portion of CN VI. Yonghong et al. [34] also found a hypoplastic CN VI in a patient with type 2 DRS. However, their image of CN VI before it was abruptly cut off in the cistern appeared to have a normal diameter compared to the contralateral side (serial images were not shown). Yang et al. [35] gathered 31 more patients with type 3 DRS and found that CN VI was absent or hypoplastic in 31 eyes (91%) and present in three eyes (9%). Patients with CN VI showed more adduction limitation than those without CN VI [35]. DRS without CN VI could be familial [36].
MRI could be very helpful for the diagnosis of DRS as follows. Firstly, patients with DRS can be misdiagnosed with traumatic CN VI palsy if abduction limitation is found after head trauma associated with traffic accident [37]. Secondly, the diagnosis of DRS could be more challenging in children if they only show abduction limitation [38]. Thirdly, patients with DRS can suddenly develop diplopia for the first time later in life and could be misdiagnosed with CN VI palsy or Miller-Fisher syndrome [39]. Lastly, patients with DRS can show atypically variable eye movements [40]. A patient without CN VI showed limited adduction and abduction most of the time, but he could fully abduct the eye with maximal effort, in contrast to most patients with DRS [40]. In patients who did not previously recognize abnormal eye movement, a diagnosis of DRS could be challenging. A patient with DRS might show a severely discrepant abduction limitation between upgaze and downgaze [41]. A patient might show coexistence of different types of DRS and could be misdiagnosed with congenital horizontal gaze palsy [42]. MRI could be very helpful for the differential diagnosis of abduction limitation with such abnormal atypical eye movements.
Okanobu et al. [25] reported the lateral rectus split into superior and inferior portions in the posterior orbit in two (40%) of five patients with DRS.
Duane radial ray syndrome is the dominant association of uni-/bilateral DRS with uni-/bilateral dysplasia of thumb, radial bone and artery, and hypoplasia of pectoral muscle. It can result from heterozygous SALL4 mutations [43]. MRI in five patients with Duane radial ray syndrome showed intraorbital and intracranial small to absent CN VI and normal intracranial CN III, optic nerve, and extraocular muscles [43]. In some orbits, a CN III branch closely approximates the lateral rectus [43].
Möbius syndrome is a complex abnormal development of the lower brainstem resulting in congenital abduction deficit, orofacial and limb malformations, musculoskeletal defects, and mental retardation [44]. Ouanounou et al. [45] reported a hypoplastic dorsal pons corresponding to the location of the facial colliculus and CN VI complexes, absent middle cerebellar peduncles, prominent superior cerebellar peduncles, small internal auditory canals, and a normal supratentorial brain. Pedraza et al. [46] emphasized straightening of the fourth ventricle floor caused by loss of the facial colliculi. Dooley et al. [47] reported calcification in the floor of the fourth ventricle. Pandey et al. [48] and Verzijl et al. [49] reported an absent CN VII. Fons-Estupina et al. [50] found compromised CN VII (100%), VI (85%), XII (40%), VIII (25%), or IX (60%) in 20 patients with Möbius syndrome. Dumars et al. [51] reported three patients with congenital complete ophthalmoplegia, ptosis, and facial diplegia. MRI showed markedly hypoplastic extraocular muscles, intraorbital motor nerves, and posterior bony orbit [51]. However, CN III, VI, VII, and VIII were normal [51]. Jiao et al. [52] also found bilaterally absent CN VI, VII, and IX in one patient and bilaterally absent CN VI and VII in another patient with Möbius syndrome. Park et al. [53] reported a patient with coexisting bilateral Duane retraction syndrome and unilateral Möbius syndrome resulting in horizontal gaze palsy and unilateral facial palsy. They found absent bilateral CN VI and absent unilateral CN VII on the side with Möbius syndrome [53]. Some patients may show bilateral absence of CN VI and VII (Fig. 6A and 6B).
Congenital fibrosis of the extraocular muscles (CFEOM) is characterized by congenital restrictive ophthalmoplegia, more severe in vertical eye movement, with or without ptosis. CFEOM can be categorized into eight complex strabismus of CFEOM1A, CFEOM1B, CFEOM2, CFEOM3A, CFEOM3B, CFEOM3C, CFEOM4 (Tukel syndrome), and CFEOM5 [5455565758596061]. CFEOM1 is diagnosed if all affected family members show the classic phenotype with bilateral involvement [5455]. CFEOM2 is characterized by bilateral ptosis and fixed exotropia with autosomal recessive inheritance and ARIX gene mutation on chromosome 11q13 [56]. CFEOM3 is a genetically as well as phenotypically heterogeneous disorder with variable clinical features. Such patient can show more unilateral involvement, can raise the eyes above midline, or might not have ptosis [5455].
Kim and Hwang [4] reported medial and/or superior rectus muscle atrophy, bilateral hypoplasia of CN III, and absent CN VI in two patients with CFEOM and synergistic divergence (Fig. 7A+7C). Because CN VI was absent only on the side of synergistic divergence, they suggested that hypoplasia of CN III is related with CFEOM. Bosley et al. [58] also reported similar findings of absent CN III and atrophy of the levator palpebrae superioris, medial/superior/inferior recti, and inferior oblique muscles. Demer et al. [759] reported hypoplastic or absent CN III and VI in the orbit, misdirection of the inferior division of CN III to the lateral rectus muscle, reduced cross section of optic nerves, severe atrophy of levator palpebrae superioris and superior recti, and variable degree of atrophy of other extraocular muscles in CFEOM1 associated with mutations in six patients with CFEOM with KIF21A mutation. Lim et al. [8] assessed the subarachnoid portions of CN III. Mean CN III diameter in CFEOM was 1.14 mm, significantly smaller than in normal subjects (mean, 2.01 mm). Demer et al. [7] evaluated 13 CFEOM3 patients with TUBB3 mutations and found variable asymmetric levator palpebrae superioris and superior rectus atrophy correlated with ptosis, limited supraduction, and small orbital motor nerves. Extraocular muscles showed variable hypoplasia, except the superior oblique muscle. They suggested that CFEOM3 phenotype is more variable than that of CFEOM1 and is often asymmetric. In addition, lateral rectus muscle misinnervation by a branch of CN III is a common feature of DRS and CFEOM1.
Some patients with CFEOM have central nervous system malformations, including agenesis of the corpus callosum, brainstem atrophy, cerebellar hemisphere atrophy, absence of the cerebral peduncle in the midbrain, colpocephaly, hypoplasia of the cerebellar vermis, expansion of the ventricular system, pachygyria, encephalocele, and/or hydrancephaly. Pieh et al. [60] reported colpocephaly, pachygyria, corpus callosum agenesis, cerebellar vermis hypoplasia, encephalocele, and/or hydrancephaly in four patients with CFEOM. Harissi-Dagher et al. [61] reported atrophy of the cerebellar hemisphere, brainstem, thalamus involving the lateral geniculate body, and the optic radiation.
In summary, selective CN III hypoplasia and corresponding extraocular muscle hypoplasia are the key imaging features of CFEOM.
Synergistic divergence is an adduction deficit with bilateral simultaneous abduction on attempted gaze to the action field of the medial rectus muscle. There have been no pathologic reports of synergistic divergence. Kim and Hwang [4] reported MRI findings of two patients with CFEOM and synergistic divergence as an absent CN VI on the side of synergistic divergence and bilateral hypoplastic CN III on the side of CFEOM. In addition, they found atrophy of the medial rectus muscle and/or superior rectus muscles [4]. Both patients showed a normal size of the inferior rectus muscle [4]. Park et al. [62] reported orbital mass and atrophy of the inferior and the medial rectus muscles in an 11-month-old girl with unilateral enophthalmos, restricted eye movement, and synergistic divergence. Pathologic examination of the orbital mass showed choroid plexus, arachnoid tissue, dura mater, and brain parenchyma. Jiao et al. [52] found an enlarged branch of the inferior division of CN III to the medial rectus muscle and possibly absent branch to the inferior rectus muscle. Jiao et al. [52] did not mention the size of the medial rectus muscle, and their figure did not show any significant atrophy of the medial rectus muscle. Oystreck et al. [63] reported 30% of maximum cross sectional size of the medial rectus muscle compared to the contralateral side in three patients with synergistic divergence. They mentioned that CN III was present in one patient.
A patient can show either normal adduction or synergistic divergence in addition to restricted eye movement compatible with CFEOM. Kim and Hwang [64] reported one such patient and the MRI findings of bilateral hypoplasia of CN III and absent CN VI on the affected side of synergistic divergence. In addition, bilateral medial and superior rectus muscles were atrophic.
Synergistic convergence is an abduction deficit with bilateral simultaneous adduction on attempted gaze to the action field of the lateral rectus muscle. Since Kim and Hwang [65] first reported such eye movement in a patient with CFEOM, there have been many reports not only with CFEOM, but also with horizontal gaze palsy with progressive scoliosis and brain stem dysplasia [666768].
Kim and Hwang [65] first reported bilateral absent CN VI and hypoplastic CN III in a patient with bilateral synergistic convergence and CFEOM. In addition, bilateral superior rectus muscles were severely atrophic, and bilateral medial rectus muscles were mildly atrophic [65]. Pieh et al. [66] reported a patient with bilateral synergistic convergence and full vertical eye movement. They found slightly hypoplastic CN III, normal CN VI, and normal extraocular muscles. Khan et al. [67] reported a similar patient with bilateral synergistic convergence and full vertical eye movement with kyphoscoliosis, in whom they found anterior midline cleft in the pons and medulla and small pons and cerebellar peduncles typical of horizontal gaze palsy and progressive scoliosis. CN III and VI and all extraocular muscles were normal in size [67]. Jain et al. [68] reported a similar patient with bilateral synergistic convergence and full vertical eye movement and kyphoscoliosis. MRI showed normal CN III, VI, and all extraocular muscles. In addition, there was a variable degree of deep midline cleft of the pons, known as the split pons sign.
In summary, the imaging findings of CCDDs are as follows.
Congenital CN III palsy:
Congenital CN IV palsy:
Duane retraction syndrome (congenital cranial dysinnervation syndrome related with CN VI):
Möbius syndrome:
Congenital fibrosis of extraocular muscles:
Synergistic divergence and synergistic convergence:
Figures and Tables
 | Fig. 1Normal cranial nerve (CN) III and VI. (A) Axial magnetic resonance image at the level of the lower midbrain shows the cisternal segments of CN III (arrows) as linear dark structures coursing in the anterolateral direction toward the cavernous sinus. (B) Axial magnetic resonance image at the pontomedullary junction shows the cisternal segments of CN VI (black arrows) coursing in the anterosuperior direction toward Dorello's canal. CN VII (double arrow) and CN VIII (white arrows) are also well identified. |
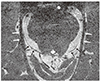 | Fig. 2Normal cranial nerve IV. Axial magnetic resonance image at the level of the inferior colliculus shows the cisternal segments of cranial nerve IV (thin arrows) exiting from the posterior aspect of the brainstem and coursing in the anterolateral direction toward the tentorium (thick arrows). |
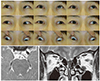 | Fig. 3Congenital cranial nerve (CN) III agenesis. (A) Ocular versions show limited elevation, adduction, and depression of the right eye. (B) Axial magnetic resonance image shows normal left CN III (arrow). Right CN III is not observed. (C) Orbital coronal magnetic resonance image shows atrophy of the right medial rectus and right inferior rectus (arrows). |
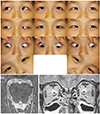 | Fig. 4Congenital cranial nerve (CN) IV agenesis. (A) Ocular versions show right hypertropia and limited depression both in downgaze and adduction in the right eye. (B) Axial magnetic resonance image shows normal left CN IV (arrows). Right CN IV is not identified. (C) Orbital coronal magnetic resonance image shows atrophy of the right superior oblique muscle (arrow). Compare with the left superior oblique (double arrow). |
 | Fig. 5Duane's retraction syndrome type 1. (A) Ocular versions show limitations of adduction and abduction and fissure narrowing on adduction in the left eye. (B) Axial magnetic resonance image at the pontomedullary junction shows normal right cranial nerve VI (arrow). Left cranial nerve VI is not identified. |
 | Fig. 6Bilateral Möbius syndrome. (A) Ocular versions show limitation on abduction in both eyes. (B) Axial magnetic resonance image at the lower pons shows normal cranial nerve VIII bilaterally (white and black arrows). Cranial nerve VI and VII are not observed bilaterally. Refer to normal VI and VII in Fig. 1B. |
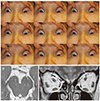 | Fig. 7Congenital fibrosis of the extraocular muscles in both eyes. (A) Ocular versions show limited ductions of both eyes. (B) Axial magnetic resonance image shows bilateral small-sized cranial nerve III (arrows), suggesting hypoplasia. (C) Orbital coronal magnetic resonance image shows mild atrophy of the right superior rectus and right medial rectus and severe atrophy of the left superior rectus and left medial rectus (arrows). |
References
1. Gutowski NJ, Bosley TM, Engle EC. 110th ENMC International Workshop: the congenital cranial dysinnervation disorders (CCDDs). Naarden, The Netherlands, 25–27 October, 2002. Neuromuscul Disord. 2003; 13:573–578.
2. Kim JH, Hwang JM. Presence of the abducens nerve according to the type of Duane's retraction syndrome. Ophthalmology. 2005; 112:109–113.
3. Ciftci E, Anik Y, Arslan A, et al. Driven equilibrium (drive) MR imaging of the cranial nerves V-VIII: comparison with the T2-weighted 3D TSE sequence. Eur J Radiol. 2004; 51:234–240.
4. Kim JH, Hwang JM. Hypoplastic oculomotor nerve and absent abducens nerve in congenital fibrosis syndrome and synergistic divergence with magnetic resonance imaging. Ophthalmology. 2005; 112:728–732.
5. Choi BS, Kim JH, Jung C, Hwang JM. High-resolution 3D MR imaging of the trochlear nerve. AJNR Am J Neuroradiol. 2010; 31:1076–1079.
6. Yang HK, Kim JH, Hwang JM. Congenital superior oblique palsy and trochlear nerve absence: a clinical and radiological study. Ophthalmology. 2012; 119:170–177.
7. Demer JL, Ortube MC, Engle EC, Thacker N. High-resolution magnetic resonance imaging demonstrates abnormalities of motor nerves and extraocular muscles in patients with neuropathic strabismus. J AAPOS. 2006; 10:135–142.
8. Lim KH, Engle EC, Demer JL. Abnormalities of the oculomotor nerve in congenital fibrosis of the extraocular muscles and congenital oculomotor palsy. Invest Ophthalmol Vis Sci. 2007; 48:1601–1606.
9. Kim JH, Hwang JM. Magnetic resonance imaging in three patients with congenital oculomotor nerve palsy. Br J Ophthalmol. 2009; 93:1266–1267.
10. Hamed LM. Associated neurologic and ophthalmologic findings in congenital oculomotor nerve palsy. Ophthalmology. 1991; 98:708–714.
11. Good WV, Barkovich AJ, Nickel BL, Hoyt CS. Bilateral congenital oculomotor nerve palsy in a child with brain anomalies. Am J Ophthalmol. 1991; 111:555–558.
12. Sun CC, Kao LY. Unilateral congenital third cranial nerve palsy with central nervous system anomalies: report of two cases. Chang Gung Med J. 2000; 23:776–781.
13. Schulz E, Jung H. Unilateral congenital oculomotor nerve palsy, optic nerve hypoplasia and pituitary malformation: a preliminary report. Strabismus. 2001; 9:33–35.
14. Kau HC, Tsai CC, Ortube MC, Demer JL. High-resolution magnetic resonance imaging of the extraocular muscles and nerves demonstrates various etiologies of third nerve palsy. Am J Ophthalmol. 2007; 143:280–287.
15. Sato M. Magnetic resonance imaging and tendon anomaly associated with congenital superior oblique palsy. Am J Ophthalmol. 1999; 127:379–387.
16. Demer JL, Clark RA, Kono R, et al. A 12-year, prospective study of extraocular muscle imaging in complex strabismus. J AAPOS. 2002; 6:337–347.
17. Kim JH, Hwang JM. MR imaging of familial superior oblique hypoplasia. Br J Ophthalmol. 2010; 94:346–350.
18. Kim JH, Hwang JM. Absence of the trochlear nerve in patients with superior oblique hypoplasia. Ophthalmology. 2010; 117:2208–2213. 2213.e1–2213.e2.
19. Lee DS, Yang HK, Kim JH, Hwang JM. Morphometry of the trochlear nerve and superior oblique muscle volume in congenital superior oblique palsy. Invest Ophthalmol Vis Sci. 2014; 55:8571–8575.
20. Yang HK, Lee DS, Kim JH, Hwang JM. Association of superior oblique muscle volumes with the presence or absence of the trochlear nerve on high-resolution MR imaging in congenital superior oblique palsy. AJNR Am J Neuroradiol. 2015; 36:774–778.
21. Kim JH, Hwang JM. Usefulness of magnetic resonance imaging in a patient with diplopia after cataract surgery. Graefes Arch Clin Exp Ophthalmol. 2012; 250:151–153.
22. Lee S, Kim SH, Yang HK, et al. Imaging demonstration of trochlear nerve agenesis in superior oblique palsy emerging during the later life. Clin Neurol Neurosurg. 2015; 139:269–271.
23. Yang HK, Kim JH, Kim JS, Hwang JM. Absent trochlear nerve with transient diplopia. Neurol Sci. 2014; 35:935–937.
24. Yang HK, Kim JH, Hwang JM. Absent trochlear nerve with contralateral superior oblique underaction. Graefes Arch Clin Exp Ophthalmol. 2013; 251:2297–2298.
25. Okanobu H, Kono R, Miyake K, Ohtsuki H. Splitting of the extraocular horizontal rectus muscle in congenital cranial dysinnervation disorders. Am J Ophthalmol. 2009; 147:550–556.e1.
26. Duane A. Congenital deficiency of abduction associated with impairment of adduction, retraction movements, contraction of palpebral fissure and oblique movements of the eye. Arch Ophthalmol. 1996; 114:1255–1256.
27. Huber A. Electrophysiology of the retraction syndromes. Br J Ophthalmol. 1974; 58:293–300.
28. Hotchkiss MG, Miller NR, Clark AW, Green WR. Bilateral Duane's retraction syndrome: a clinical-pathologic case report. Arch Ophthalmol. 1980; 98:870–874.
29. Mulhern M, Keohane C, O'Connor G. Bilateral abducens nerve lesions in unilateral type 3 Duane's retraction syndrome. Br J Ophthalmol. 1994; 78:588–591.
30. Parsa CF, Grant PE, Dillon WP Jr, et al. Absence of the abducens nerve in Duane syndrome verified by magnetic resonance imaging. Am J Ophthalmol. 1998; 125:399–401.
31. Kim JH, Hwang JM. Abducens nerve is present in patients with type 2 Duane's retraction syndrome. Ophthalmology. 2012; 119:403–406.
32. Denis D, Dauletbekov D, Alessi G, et al. Duane retraction syndrome: MRI features in two cases. J Neuroradiol. 2007; 34:137–140.
33. Denis D, Dauletbekov D, Girard N. Duane retraction syndrome: type II with severe abducens nerve hypoplasia on magnetic resonance imaging. J AAPOS. 2008; 12:91–93.
34. Yonghong J, Kanxing Z, Zhenchang W, et al. Detailed magnetic resonance imaging findings of the ocular motor nerves in Duane's retraction syndrome. J Pediatr Ophthalmol Strabismus. 2009; 46:278–285.
35. Yang HK, Kim JH, Hwang JM. Abducens nerve in patients with type 3 Duane's retraction syndrome. PLoS One. 2016; 11:e0150670.
36. Kim JH, Hwang JM. MR imaging diagnosis of familial Duane's retraction syndrome by documentation of the absence of the abducens nerves. Eye (Lond). 2007; 21:1431–1433.
37. Kim JH, Hwang JM. Magnetic resonance imaging in patients with abduction deficit found after head trauma. J Neurol. 2005; 252:224–226.
38. Kim JH, Hwang JM. Usefulness of MR imaging in children without characteristic clinical findings of Duane's retraction syndrome. AJNR Am J Neuroradiol. 2005; 26:702–705.
39. Kim JH, Kim JS, Hwang JM. Duane's retraction syndrome as a cause of diplopia in adults. Eur J Neurol. 2008; 15:e25–e27.
40. Kim JH, Hwang JM. Abducens nerve in a patient with Duane retraction syndrome. Can J Ophthalmol. 2014; 49:e52–e54.
41. Kim JH, Hwang JM. A discrepant abduction as an additional characteristic finding in a patient with Duane's retraction syndrome. J Neurol. 2009; 256:1920–1921.
42. Kim JH, Kim JS, Hwang JM. Coexistence of different types of Duane's retraction syndrome manifesting as a congenital gaze palsy. J Neurol. 2006; 253:390–391.
43. Demer JL, Clark RA, Lim KH, Engle EC. Magnetic resonance imaging of innervational and extraocular muscle abnormalities in Duane-radial ray syndrome. Invest Ophthalmol Vis Sci. 2007; 48:5505–5511.
44. Verzijl HT, van der Zwaag B, Cruysberg JR, Padberg GW. Mobius syndrome redefined: a syndrome of rhombencephalic maldevelopment. Neurology. 2003; 61:327–333.
45. Ouanounou S, Saigal G, Birchansky S. Mobius syndrome. AJNR Am J Neuroradiol. 2005; 26:430–432.
46. Pedraza S, Gamez J, Rovira A, et al. MRI findings in Mobius syndrome: correlation with clinical features. Neurology. 2000; 55:1058–1060.
47. Dooley JM, Stewart WA, Hayden JD, Therrien A. Brainstem calcification in Mobius syndrome. Pediatr Neurol. 2004; 30:39–41.
48. Pandey PK, Shroff D, Kapoor S, et al. Bilateral incyclotorsion, absent facial nerve, and anotia: fellow travelers in Mobius sequence or oculoauriculovertebral spectrum? J AAPOS. 2007; 11:310–312.
49. Verzijl HT, Valk J, de Vries R, Padberg GW. Radiologic evidence for absence of the facial nerve in Mobius syndrome. Neurology. 2005; 64:849–855.
50. Fons-Estupina MC, Poo P, Colomer J, Campistol J. Moebius sequence: clinico-radiological f indings. Rev Neurol. 2007; 44:583–588.
51. Dumars S, Andrews C, Chan WM, et al. Magnetic resonance imaging of the endophenotype of a novel familial Mobius-like syndrome. J AAPOS. 2008; 12:381–389.
52. Jiao YH, Zhao KX, Wang ZC, et al. Magnetic resonance imaging of the extraocular muscles and corresponding cranial nerves in patients with special forms of strabismus. Chin Med J (Engl). 2009; 122:2998–3002.
53. Park C, Kim JH, Hwang JM. Coexistence of Mobius syndrome and Duane's retraction syndrome. Graefes Arch Clin Exp Ophthalmol. 2012; 250:1707–1709.
54. Engle EC, Goumnerov BC, McKeown CA, et al. Oculomotor nerve and muscle abnormalities in congenital fibrosis of the extraocular muscles. Ann Neurol. 1997; 41:314–325.
55. Heidary G, Engle EC, Hunter DG. Congenital fibrosis of the extraocular muscles. Semin Ophthalmol. 2008; 23:3–8.
56. Engle EC, Wang SM, Zwaan JT, et al. Linkage and homozygosity mapping of a variant of congenital fibrosis of the extraocular muscles to chromosome 11q13. 1. Am J Hum Genet. 1997; 61:30.
57. Yamada K, Andrews C, Chan WM, et al. Heterozygous mutations of the kinesin KIF21A in congenital fibrosis of the extraocular muscles type 1 (CFEOM1). Nat Genet. 2003; 35:318–321.
58. Bosley TM, Salih MA, Alorainy IA, et al. Clinical characterization of the HOXA1 syndrome BSAS variant. Neurology. 2007; 69:1245–1253.
59. Demer JL, Clark RA, Engle EC. Magnetic resonance imaging evidence for widespread orbital dysinnervation in congenital fibrosis of extraocular muscles due to mutations in KIF21A. Invest Ophthalmol Vis Sci. 2005; 46:530–539.
60. Pieh C, Goebel HH, Engle EC, Gottlob I. Congenital fibrosis syndrome associated with central nervous system abnormalities. Graefes Arch Clin Exp Ophthalmol. 2003; 241:546–553.
61. Harissi-Dagher M, Dagher JH, Aroichane M. Congenital fibrosis of the extraocular muscles with brain-stem abnormalities: a novel finding. Can J Ophthalmol. 2004; 39:540–545.
62. Park KA, Kim HJ, Kim YD. Ectopic brain in the orbit with congenital adduction deficit and simultaneous abduction. Ophthal Plast Reconstr Surg. 2007; 23:244–246.
63. Oystreck DT, Khan AO, Vila-Coro AA, et al. Synergistic divergence: a distinct ocular motility dysinnervation pattern. Invest Ophthalmol Vis Sci. 2009; 50:5213–5216.
64. Kim JH, Hwang JM. Variable synergistic divergence. Optom Vis Sci. 2009; 86:1386–1388.
65. Kim JH, Hwang JM. Adduction on attempted abduction: the opposite of synergistic divergence. Arch Ophthalmol. 2006; 124:918–920.
66. Pieh C, Berlis A, Lagreze WA. Synergistic convergence in congenital extraocular muscle misinnervation. Arch Ophthalmol. 2008; 126:574–576.
67. Khan AO, Oystreck DT, Al-Tassan N, et al. Bilateral synergistic convergence associated with homozygous ROB03 mutation (p.Pro771Leu). Ophthalmology. 2008; 115:2262–2265.
68. Jain NR, Jethani J, Narendran K, Kanth L. Synergistic convergence and split pons in horizontal gaze palsy and progressive scoliosis in two sisters. Indian J Ophthalmol. 2011; 59:162–165.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download