This article has been
cited by other articles in ScienceCentral.
Abstract
Purpose
The purpose of this study was to investigate the clinical efficacy of an optimized prolate ablation procedure for correcting residual refractive errors following laser surgery.
Methods
We analyzed 24 eyes of 15 patients who underwent an optimized prolate ablation procedure for the correction of residual refractive errors following laser in situ keratomileusis, laser-assisted subepithelial keratectomy, or photorefractive keratectomy surgeries. Preoperative ophthalmic examinations were performed, and uncorrected distance visual acuity, corrected distance visual acuity, manifest refraction values (sphere, cylinder, and spherical equivalent), point spread function, modulation transfer function, corneal asphericity (Q value), ocular aberrations, and corneal haze measurements were obtained postoperatively at 1, 3, and 6 months.
Results
Uncorrected distance visual acuity improved and refractive errors decreased significantly at 1, 3, and 6 months postoperatively. Total coma aberration increased at 3 and 6 months postoperatively, while changes in all other aberrations were not statistically significant. Similarly, no significant changes in point spread function were detected, but modulation transfer function increased significantly at the postoperative time points measured.
Conclusions
The optimized prolate ablation procedure was effective in terms of improving visual acuity and objective visual performance for the correction of persistent refractive errors following laser surgery.
Go to :

Keywords: Optimized prolate ablation, Refractive surgery, Residual refractive errors
Laser
in situ keratomileusis (LASIK), laser-assisted subepithelial keratectomy (LASEK), and photorefractive keratectomy (PRK) are effective surgical techniques for the correction of myopia, myopic astigmatism, and mild to moderate hypermetropia. Although the predictability of these techniques has improved, the precise refractive outcome is not always guaranteed because of different responses of living tissue to laser ablation [
1]. The reported rates of myopic regression that require retreatment ranged from 6% to 14% for up to 1 year following the surgical procedure, and from 20% to 27% for longer follow-up periods [
23]. However, recent studies have indicated that the rates have declined [
45]. Higher initial corrections, the degree of astigmatism, and advanced age of patients are known to increase the odds of requiring retreatment [
6]. Although not all of the mechanisms involved in myopic regression have been elucidated, the number of patients who require retreatment for myopic regression after primary refractive surgery has decreased. The reported decrease has been attributed to an enlarged optical zone, the development of laser technology, and improved postoperative wound management. However, some patients still experienced myopic regression after the performance of primary refractive surgery. Further, retreatment planning following primary refractive surgery is dependent on the individual needs of each patient.
Retreatment tends to be less predictable compared with primary ablation because of difficulties related to analysis of the refractive state, unpredictable rates of wound healing, and corneal irregularities induced by previous ablation procedures. Previous studies have reported that the combination of corneal wavefront and topographic aspheric treatments has been used successfully to treat eyes that have undergone previous refractive surgeries [
57].
Optimized prolate ablation (OPA) employs wavefront aberrometry and corneal topography to treat preexisting spherical aberrations and to maintain the preoperative corneal asphericity (Q value) [
89]. As improved outcomes had been achieved in previous studies with the combined application of the corneal wavefront and topographic aspheric treatments, we endeavored to assess clinical outcomes following application of the OPA retreatment method. In the current study, we investigated the clinical efficacy of an OPA procedure for the treatment of persistent refractive errors following previous refractive surgery.
Materials and Methods
In this retrospective study, we evaluated patients who had undergone retreatment OPA following previous LASIK, LASEK, or PRK surgery. The study was conducted in accordance with the Declaration of Helsinki, and surgical procedures were conducted after all patients participated in a thorough preoperative discussion of the risks and benefits of OPA and informed consent was obtained.
Inclusion criteria for patients who had undergone previous LASIK, LASEK, or PRK between 1997 and 2012 included an age greater than 20 years and myopia measurements less than or equal to –3.50 manifest refraction spherical equivalent (MRSE) diopters (D). Patients who had active systemic ocular disease or who underwent previous ocular surgery other than the aforementioned primary refractive surgeries were excluded from the study. All patients were symptomatic and had residual refractive errors.
All patients underwent a preoperative ophthalmic evaluation, and uncorrected distance visual acuity (UDVA), corrected distance visual acuity (CDVA), manifest refraction, corneal topography, wavefront aberrometry, modulation transfer function (MTF), point spread function (PSF), slit-lamp biomicroscopy, tonometry, and fundus measurements were obtained postoperatively at 1, 3, and 6 months. Corneal haze was assessed on a scale from 0 to 4 according to the method proposed by Fantes et al. [
10]. Corneal topography and wavefront aberrometry were measured on the same optical axis using an OPD-Scan III wavefront aberrometer (Nidek, Tokyo, Japan), following the application of tropicamide 0.5%-phenylephrine 0.5% eye drops (Mydrin-P; Santen, Osaka, Japan) and the subsequent dilation of the pupil to 6.0 mm. All wavefront measurements were performed to the eighth Zernike order. The device software separated corneal and internal aberrations to evaluate the effects of the optical elements in the visual system. Corneal asphericity (Q value) was measured by software-simulated corneal topography. The objective visual quality was assessed using an MTF graph, which revealed the degree of contrast transfer at different spatial frequencies by calculating the area ratio and the ratio of the area under the MTF graph covered by the vertical and horizontal axes to the area under the normal eye curve. PSF was also calculated using the Strehl ratio, which is the ratio of the PSF value to the theoretical diffraction limit.
All patients underwent treatment by one surgeon (BJC) using the EC 5000 CXII excimer laser platform (Nidek). Manifest refraction, corneal topographic data, and wavefront data obtained from the OPD-Scan III were considered when designing the ablation procedure. All data were transferred to the Optimized Prolate Ablation Software (ver. 1.00, Nidek) for treatment planning. The ablation design was established automatically using the software, by setting the value of the postoperative ocular spherical aberration to zero. The software did not target to correct ocular coma and trefoil aberration. The surgeon adjusted the degree of refractive correction for age, pretreatment manifest refraction, and CDVA. The eyes of each patient were prepared in a sterile fashion, and a topical anesthetic (proparacaine hydrochloride 0.5%, Alcaine; Alcon, Fort Worth, TX, USA) was instilled. Following the application of the Carones LASEK Pump OZ Chamber (9.0 mm in diameter; ASCIO, Copenhagen, Denmark), the cone was filled with 20% alcohol solution mixed with Liquifilm tears (Polyvinyl Alcohol 1.4%; Allergan, Irvine, CA, USA). The alcohol solution was flushed out for 40 seconds after instillation using a cold balanced salt solution. To detect torsion error, the image of the patient's iris was compared with the image acquired using the OPD-Scan III device. If the torsion error was greater than 2°, the patient's head was repositioned in an attempt to decrease the error. The optical zone was established using the Optimized Prolate Ablation software, and covered the entire scotopic pupil diameter, considering the ablation depth. The transition zone was determined to be 1.5 mm larger than the optical zone diameter. The mean optical zone and transition zone were 6.3 mm and 7.9 mm, respectively. A 14-ring-shaped filter paper disc saturated with 0.02% mitomycin C was applied on the cornea for 15 seconds to prevent contact with the central portion of the cornea. Irrigation with cold balanced salt solution was performed for 15 seconds following the removal of the paper disc [
1112]. A therapeutic soft contact lens was then applied to ensure complete epithelial healing. Finally, a standardized regimen of topical steroids and antibiotics was recommended for each patient.
Postoperative UDVA and CDVA, ocular wavefront aberrations, corneal spherical aberration, corneal asphericity, MTF, and PSF were compared to preoperative (before retreatment) values using analysis of variance and Bonferroni tests. A p-value less than 0.05 was considered statistically significant. All analyses were performed using the SPSS ver. 12.0 (SPSS Inc., Chicago, IL, USA).
Go to :

Results
Patients
The study included 24 eyes from 15 enrolled patients, including eight males and seven females. The mean patient age was 33 years (range, 25 to 45 years). Twelve eyes (eight patients) had undergone a previous LASIK procedure, nine eyes (five patients) a previous LASEK procedure, and three eyes (two patients) had undergone PRK. The mean interval between the first refractive procedure and retreatment was 108 ± 60 months (range, 12 months to 20.9 years). No intraoperative or postoperative complications were detected.
Refractive and visual acuity outcomes
The mean MRSE before primary surgery was −6.67 ± 1.98 D (range, −2.75 to –10.50 D), and the mean cylinder was 0.81 ± 0.84 D (range, 0.00 to 2.58 D). In addition, the residual stromal bed measurement was 312 ± 38.3 µm (range, 271 to 378 µm). Preoperatively, and 1, 3 and 6 months post performance of the surgical procedures, the mean MRSE values were −2.18 ± 0.67 D, −0.02 ± 0.61 D, −0.19 ± 0.70 D, and −0.27 ± 0.64 D, respectively (
Table 1). The reduction in MRSE was statistically significant at all postoperative time points when compared to preoperative measurements (
p < 0.005). Postoperative astigmatism was 0.32 ± 0.26 D (range, 0.00 to 0.75 D) at 6 months, which remained statistically unchanged from preoperative astigmatism measurements of 0.30 ± 0.40 D (range, 0.00 to 1.25 D) (
Fig. 1). Postoperative changes in spherical equivalent and astigmatic error are shown in
Figs. 2 and
3, respectively. A statistically significant increase (
p < 0.001) was detected in the mean UDVA from 0.42 ± 0.29 logarithm of the minimum angle of resolution (logMAR) preoperative measurements (range, 0.10 to 1.00 logMAR) to −0.02 ± 0.08 logMAR postoperative measurements (20 / 50 to 20 / 20 as Snellen equivalent) acquired at 6 months (range, −0.18 to 0.15 logMAR). Twenty eyes (83.3%) had a UDVA of 20 / 20 or better in terms of efficacy (
Fig. 4). CDVA exhibited no statistical change between baseline (postoperative) and 6 months postoperatively. For the entire follow-up period, no eye lost two or more CDVA lines, and 23 eyes (95.8%) maintained or gained CDVA lines (
Fig. 5). Fifteen eyes (62.5%) were within ±0.50 D of attempted correction, and 20 eyes (83.3%) were within ±1.00 D 6 months postoperatively (
Fig. 6).
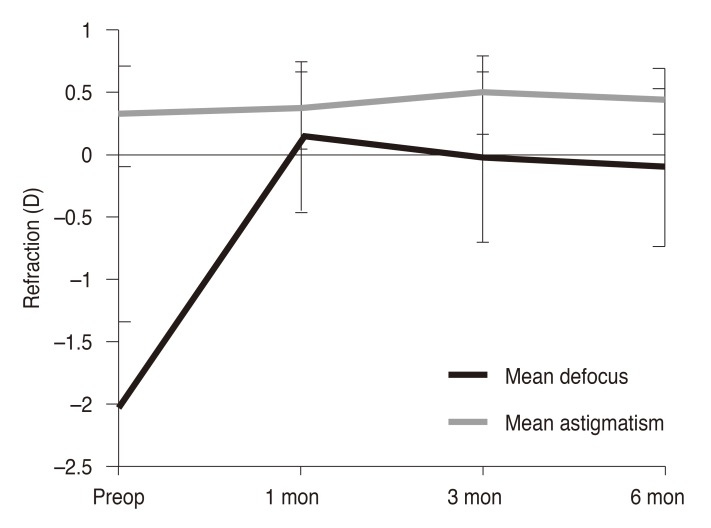 | Fig. 1Postoperative stability of refractive errors. D = diopters; Preop = preoperative.
|
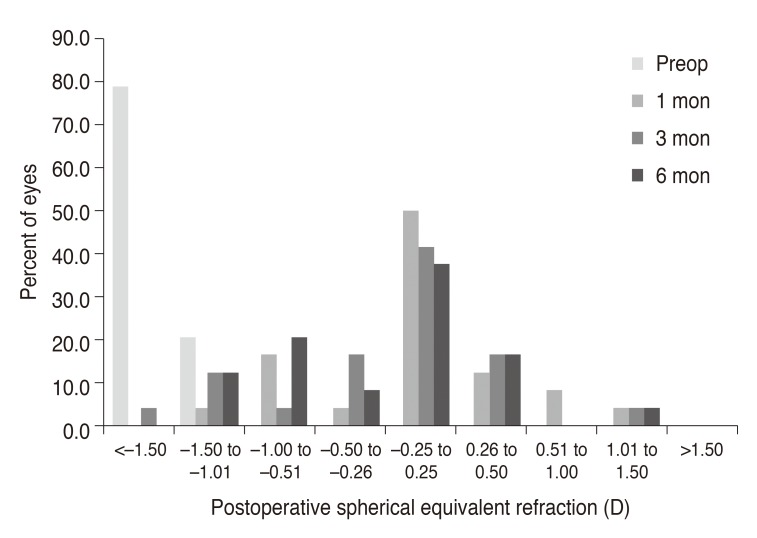 | Fig. 2Postoperative changes in spherical equivalent of manifest refraction. Preop = preoperative; D = diopters.
|
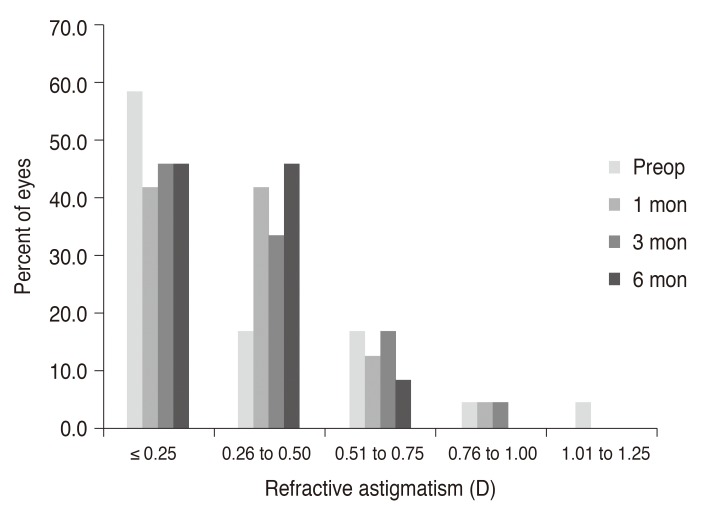 | Fig. 3Postoperative changes in spherical equivalent of manifest refraction. Preop = preoperative; D = diopters.
|
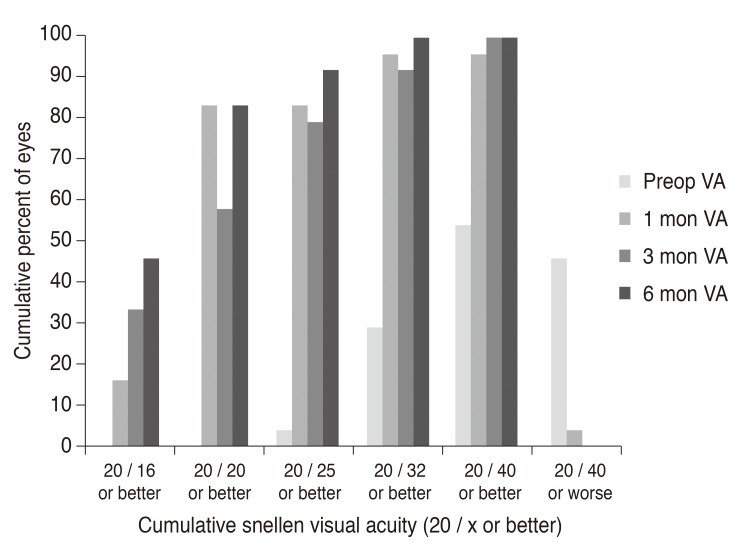 | Fig. 4Postoperative changes in astigmatic error of manifest refraction. Preop = preoperative; D = diopters.
|
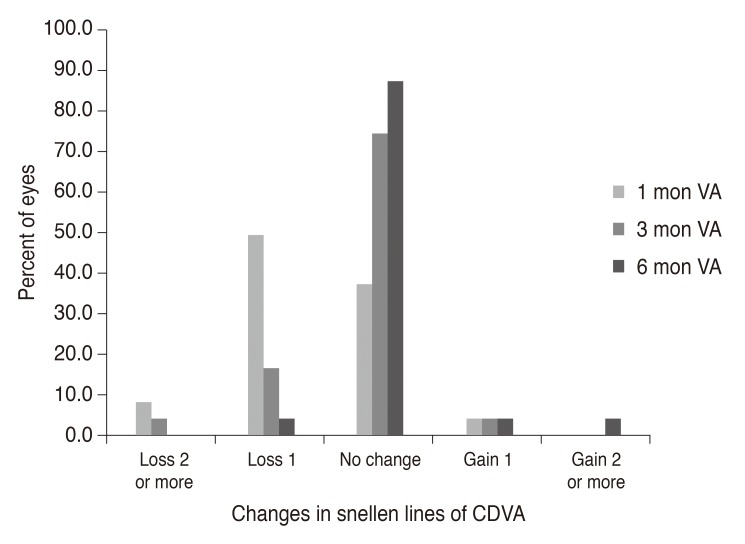 | Fig. 5Postoperative distribution of uncorrected visual acuity. Preop = preoperative; VA = visual acuity.
|
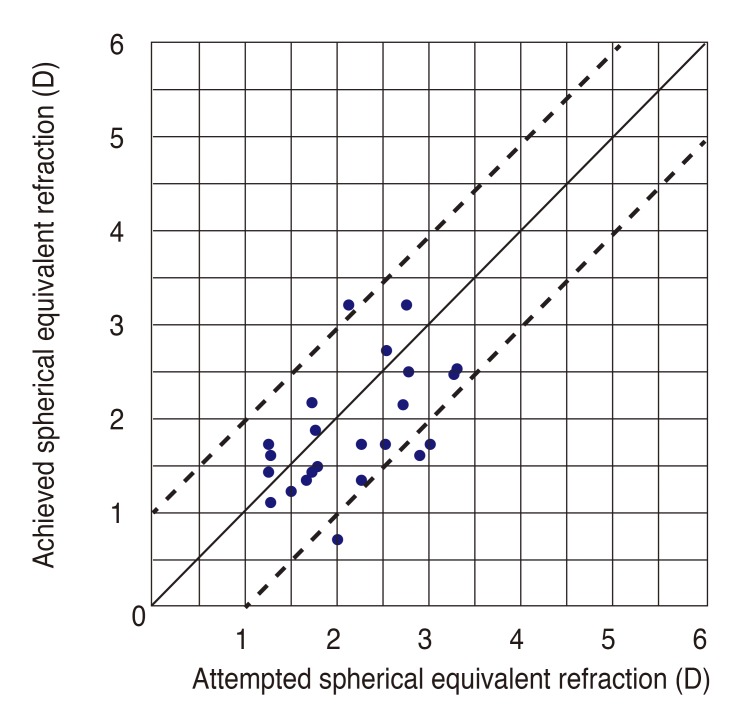 | Fig. 6Attempted versus achieved postoperative spherical equivalent. D = diopters.
|
Table 1
Preoperative and postoperative follow-up parameters
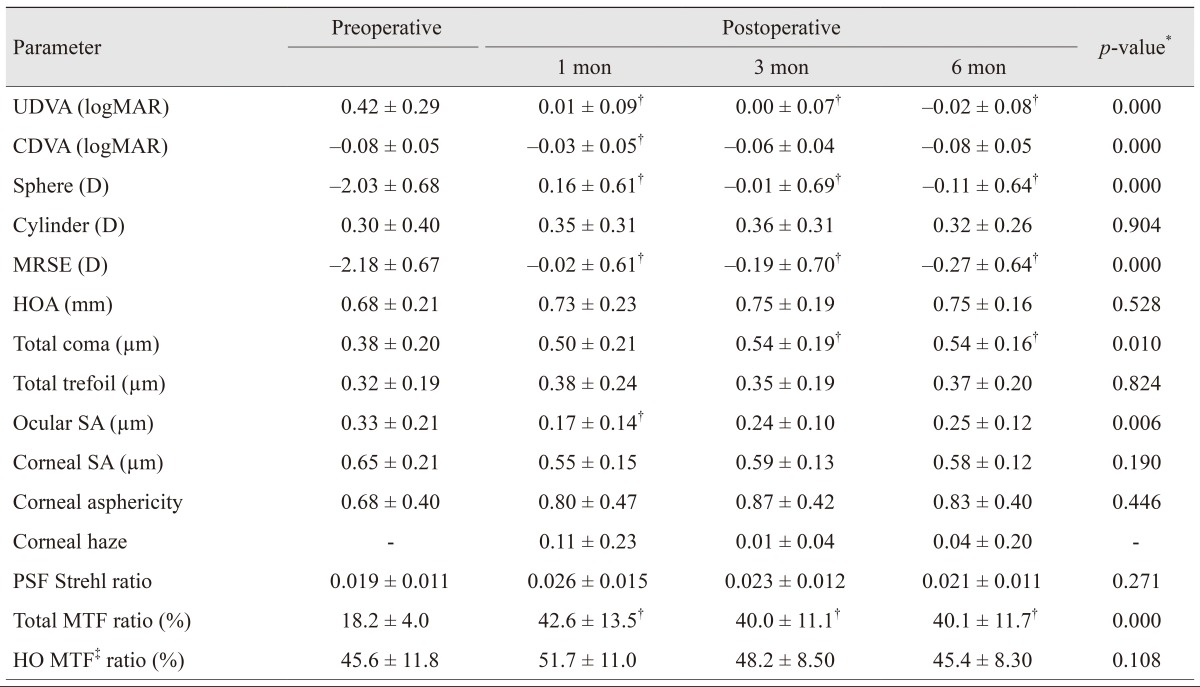

Wavefront aberrations
No significant differences were detected in the mean preoperative root mean square of ocular higher-order aberrations (HOA) after the OPA retreatment at all postoperative time points (
Fig. 7A). The mean ocular spherical aberration decreased from 0.33 ± 0.21 µm preoperatively to 0.17 ± 0.14 µm (
p < 0.005) at 1 month postoperatively, but exhibited no statistically significant postoperative change at 3 or 6 months when compared to baseline (
Fig. 7B). While the mean coma aberration increased significantly at 3 and 6 months postoperatively, no statistically significant changes were noted in the mean trefoil aberration (
Fig. 7C and 7D). Corneal spherical aberration remained unchanged at all postoperative time points (
Fig. 7B).
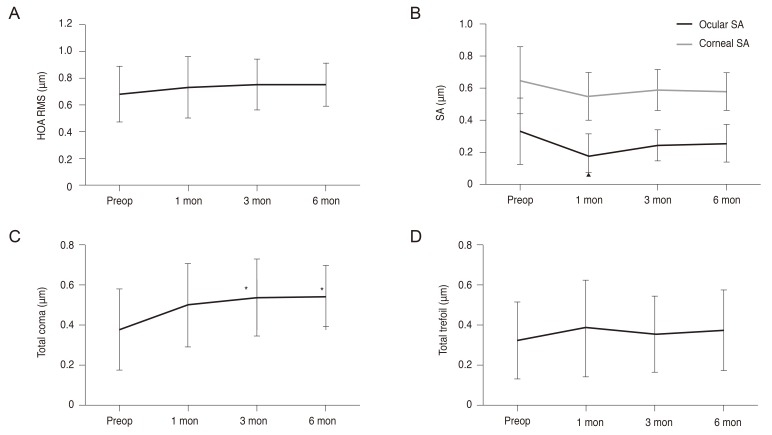 | Fig. 7Postoperative changes in ocular aberrations. (A) Total ocular HOA RMS, (B) ocular and corneal spherical aberration, (C) total coma aberrations, (D) total trefoil aberrations. HOA = higher-order aberration; RMS = root mean square; Preop = preoperative; SA = spherical aberration. *p < 0.05. All aberrations are reported for a 6.0-mm pupil plotted to the eighth Zernike order. Root-mean-square values are reported.
|
Corneal asphericity and haze
Corneal asphericity (Q value) was maintained after OPA retreatment at all postoperative time points; however, the Q value tended to increase after OPA retreatment. Corneal haze after retreatment remained minimal.
Objective visual quality
The mean MTF representing the area ratio increased significantly at all postoperative time points compared to the preoperative values (
Table 1). MTF simulated with the eye corrected for lower-order aberrations (e.g., sphere and cylinder) and the higher-order (HO) MTF exhibited no significant changes after retreatment. PSF presented as a Strehl ratio, and the ratio of the PSF value to the theoretical diffraction limit remained unchanged postoperatively.
Go to :

Discussion
The essential advantages of OPA are to minimize the induction of higher order aberrations and to maintain a prolate shape of cornea. By utilizing both wavefront aberrometry and corneal topography, reducing the induced higher order aberrations while maintaining corneal asphericity was reported to be successful, and clinical outcomes of these trials were also reported to be promising in the literature. Spherical aberrations and HOAs disturbing visual quality could be reduced [
1314]. Halo and glare were reported to also be decreased [
15].
The results of the current study indicate that OPA is an effective and predictable treatment for the correction of residual refractive errors following LASIK, LASEK, and PRK procedures. UDVA changed from 20 / 50 preoperatively to 20 / 20 postoperatively, and the spherical equivalent was significantly reduced (
p < 0.005) at all postoperative time points evaluated. Further, visual outcomes in the current study were similar or superior to those from previous investigations involving corneal wavefront retreatment [
516]. The mean refractive astigmatism remained unchanged after OPA retreatment, while the preoperative range of astigmatism decreased in the 6 months following the performance of the surgical procedure. Further studies involving the investigation of OPA retreatment for astigmatism would be helpful to assess OPA efficacy and safety in astigmatism retreatment patients.
In our study, the predictability (±0.50 D from intended refraction) was 62.5%. Other investigations have reported a predictability of 76% for wavefront-guided ablations, 91% for topography-guided ablations, and 100% when prolate ablation was applied as the primary treatment [
1317]. Compared to the application of the same OPA laser ablation pattern in virgin cornea, pretreated corneas exhibited several drawbacks. Corneal surface irregularities after primary laser treatment, or flap making and subclinical decentration may impede accurate preoperative evaluation for treatment planning and precise ablation. Moreover, altered corneal wound healing responses are likely explanations for limited OPA retreatment predictability.
In this study, corneal haze, one of the complications resulting from corneal ablation, was not apparent in any patients that underwent OPA retreatment. Corneal micro-irregularities have been reported to be one of the possible causes of corneal opacity after refractive surgery [
1819]. After laser ablation, collagen fibers newly synthesized to cover the irregular surface, are known to contribute to the development of corneal opacity, refractive errors, and the induction of HOA [
20]. In our surgical procedure, phototherapeutic keratectomy surface smoothing and application of mitomycin C might have prevented corneal opacity. Eyes treated with additional phototherapeutic keratectomy smoothing, which was performed to remove corneal micro-irregularities, were reported to be less likely to develop corneal haze and collagen fibers [
212223].
Although factors causing regression after LASEK, LASIK, and PRK are not clearly understood, previous investigations have revealed that regression might be caused by molecular memory in the corneal collagen fibers, or by stromal remodeling, corneal ectasia, corneal hydration, the effect of intraocular pressure on the thinned cornea, or by compensatory epithelial hyperplasia [
624]. Prior reports have likewise indicated that variability in corneal wound healing, including keratocyte apoptosis, biomechanical properties, and other factors might contribute to the apparent changes [
2526]. Further, compared to initial treatments, secondary laser treatments might induce atypical or unpredictable corneal wound healing.
Refractive results remained stable until 6 months after OPA retreatment. In addition, the safety of the procedure was demonstrated by the postoperative loss of no more than two CDVA lines at 6 months. In one patient, postoperative CDVA decreased from 30 / 20 to 25 / 20 in conjunction with the loss of one CDVA line at 6 months. The patient was a 34-year-old female who had undergone primary LASIK surgery 13.4 years prior to retreatment. Her refractive error measurements before the primary LASIK surgery and before the retreatment were −6.34 and −2.25, respectively. However, the patient exhibited no astigmatic error before retreatment. Six months following retreatment, her refractive error was −0.50, and her UDVA had increased from 20 / 40 to 25 / 20. While ocular and corneal spherical aberrations decreased and total MTF increased 6 months postoperatively, total HOA, trefoil, and coma aberration measurements were increased. Thus, a possible explanation for decreased CDVA might be the increase in total HOA, trefoil, and coma aberration.
The Q value, the coefficient of asphericity, is one of the coefficients used to express the conic shape factor [
2728]. However, corneal topography revealed no statistically significant changes in corneal asphericity (Q value) at any time point after OPA retreatment. Corneal spherical aberrations tended to decrease after OPA retreatment, but the changes were not statistically significant. Similarly, no statistically significant changes were detected in any of the other aberrations measured by wavefront analysis, with the exception of total coma aberration, which demonstrated significant postoperative increases at 3 and 6 months.
MTF and PSF are objective methods used to assess the quality of vision. The overall MTF exhibited significant increases at all postoperative time points. In the MTF plot of corrections for lower-order aberrations (e.g., defocus and astigmatism), no statistically significant changes were detected. Correction of defocus and astigmatism after retreatment may be attributed to the increase in total MTF. No statistically significant changes were found in HOAs, which corresponded to no indications of statistically significant changes on the HO MTF plot. Likewise, PSF, which is closely related to visual function at night, exhibited no significant changes after the retreatment.
Among higher order aberrations, coma aberration was increased while corneal asphericity and most ocular aberrations were not induced. The OPA algorithm is based on both wavefront and corneal topographic data to treat myopia, and attempts to preserve the natural shape of the cornea as much as possible. Nevertheless, worsened tear film dynamics and subclinical decentration after primary treatment can interrupt precise ablation. Further, corneal or flap surface irregularities can negatively affect the retreatment results. In addition, we used ablation design software that only corrected ocular spherical aberration. HOAs, with the exception of coma aberrations, were maintained after the retreatment, while spherical aberration decreased. Corneal asphericity was likewise maintained. Overall, the visual quality of the patient is determined by various factors including residual refractive error, higher order aberrations and corneal asphericity. Although coma aberration, one of the higher order aberrations, was increased, total MTF as an objective measurement of visual quality showed improvement in this study.
OPA treatment is aimed at minimizing the induction of spherical aberrations and maintaining the prolate corneal shape. While postoperative visual acuity is an indicator of treatment efficacy, OPA retreatment will provide successful visual recovery and optical quality improvement. In this study, corneal asphericity and most ocular aberrations were maintained successfully, which could contribute to the improved visual quality after the retreatment.
The drawbacks of the current study were the relatively small number of cases evaluated. The size of the patient group was insufficient to draw conclusions. However, considering the limited number of patients who are candidates for retreatment, we believe this study provides valuable data. Further, this is the first report of OPA retreatment for residual refractive errors following laser surgery. Finally, the results indicate that OPA retreatment provided effective and reliable surgical outcomes, and objective visual performance revealed significant improvement after retreatment.
Go to :

Acknowledgements
This study was partially supported by a grant of the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (HI14C2044).
Go to :

Notes
Go to :

References
1. Rashad KM. Laser in situ keratomileusis retreatment for residual myopia and astigmatism. J Refract Surg. 2000; 16:170–176. PMID:
10766386.

2. Hersh PS, Fry KL, Bishop DS. Incidence and associations of retreatment after LASIK. Ophthalmology. 2003; 110:748–754. PMID:
12689897.

3. Alio JL, Muftuoglu O, Ortiz D, et al. Ten-year follow-up of laser in situ keratomileusis for high myopia. Am J Ophthalmol. 2008; 145:55–64. PMID:
17996210.
4. Kanellopoulos AJ, Asimellis G. Refractive and keratometric stability in high myopic LASIK with high-frequency femtosecond and excimer lasers. J Refract Surg. 2013; 29:832–837. PMID:
24088061.

5. Aslanides IM, Kolli S, Padroni S, Arba Mosquera S. Stability of therapeutic retreatment of corneal wavefront customized ablation with the SCHWIND CAM: 4-year data. J Refract Surg. 2012; 28:347–352. PMID:
22515177.

6. Bragheeth MA, Fares U, Dua HS. Re-treatment after laser in situ keratomileusis for correction of myopia and myopic astigmatism. Br J Ophthalmol. 2008; 92:1506–1510. PMID:
18757469.

7. Toda I, Yamamoto T, Ito M, et al. Topography-guided ablation for treatment of patients with irregular astigmatism. J Refract Surg. 2007; 23:118–125. PMID:
17326350.

8. El-Danasoury A, Bains HS. Optimized prolate corneal ablation: case report of the first treated eye. J Refract Surg. 2005; 21(5 Suppl):S598–S602. PMID:
16212286.

9. El Danasoury AM, Holladay J, Waring GO 3rd, et al. A contralateral, randomized comparison of optimized prolate ablation and conventional LASIK for myopia with the NIDEK excimer laser platform. J Refract Surg. 2012; 28:453–461. PMID:
22767164.

10. Fantes FE, Hanna KD, Waring GO 3rd, et al. Wound healing after excimer laser keratomileusis (photorefractive keratectomy) in monkeys. Arch Ophthalmol. 1990; 108:665–675. PMID:
2334323.

11. Maldonado MJ. Intraoperative MMC after excimer laser surgery for myopia. Ophthalmology. 2002; 109:826. PMID:
11986073.

12. Jain S, McCally RL, Connolly PJ, Azar DT. Mitomycin C reduces corneal light scattering after excimer keratectomy. Cornea. 2001; 20:45–49. PMID:
11189003.

13. Kang EC, Choi BJ, Kim EK, Kim TI. Clinical outcomes of optimized prolate ablation and custom aspheric treatment in laser-assisted subepithelial keratectomy. J Cataract Refract Surg. 2012; 38:445–452. PMID:
22340605.

14. Choi BJ, Park YM, Lee JS. Clinical outcomes between optical path difference custom aspheric treatment and optimized prolate ablation photorefractive keratectomy in myopia exceeding 8 diopters. Eye (Lond). 2015; 29:356–362. PMID:
25397788.

15. Chayet A, Bains HS. Prospective, randomized, double-blind, contralateral eye comparison of myopic LASIK with optimized aspheric or prolate ablations. J Refract Surg. 2012; 28:112–119. PMID:
22201324.

16. Alio JL, Pinero DP, Plaza Puche AB. Corneal wavefront-guided enhancement for high levels of corneal coma aberration after laser in situ keratomileusis. J Cataract Refract Surg. 2008; 34:222–231. PMID:
18242444.
17. Kermani O, Schmiedt K, Oberheide U, Gerten G. Topographic- and wavefront-guided customized ablations with the NIDEK-EC5000CXII in LASIK for myopia. J Refract Surg. 2006; 22:754–763. PMID:
17061712.

18. Vinciguerra P, Azzolini M, Radice P, et al. A method for examining surface and interface irregularities after photorefractive keratectomy and laser in situ keratomileusis: predictor of optical and functional outcomes. J Refract Surg. 1998; 14(2 Suppl):S204–S206. PMID:
9571554.

19. Balestrazzi E, De Molfetta V, Spadea L, et al. Histological, immunohistochemical, and ultrastructural findings in human corneas after photorefractive keratectomy. J Refract Surg. 1995; 11:181–187. PMID:
7553088.
20. Oshika T, Klyce SD, Applegate RA, et al. Comparison of corneal wavefront aberrations after photorefractive keratectomy and laser in situ keratomileusis. Am J Ophthalmol. 1999; 127:1–7. PMID:
9932992.

21. Vinciguerra P, Azzolini M, Airaghi P, et al. Effect of decreasing surface and interface irregularities after photorefractive keratectomy and laser in situ keratomileusis on optical and functional outcomes. J Refract Surg. 1998; 14(2 Suppl):S199–S203. PMID:
9571553.

22. Vinciguerra P, Camesasca FI, Randazzo A. One-year results of butterfly laser epithelial keratomileusis. J Refract Surg. 2003; 19(2 Suppl):S223–S226. PMID:
12699177.

23. Serrao S, Lombardo M. One-year results of photorefractive keratectomy with and without surface smoothing using the technolas 217C laser. J Refract Surg. 2004; 20:444–449. PMID:
15523955.

24. Bas AM, Onnis R. Excimer laser in situ keratomileusis for myopia. J Refract Surg. 1995; 11(3 Suppl):S229–S233. PMID:
7553096.

25. Mohan RR, Hutcheon AE, Choi R, et al. Apoptosis, necrosis, proliferation, and myofibroblast generation in the stroma following LASIK and PRK. Exp Eye Res. 2003; 76:71–87. PMID:
12589777.

26. Roberts C. Biomechanics of the cornea and wavefront-guided laser refractive surgery. J Refract Surg. 2002; 18:S589–S592. PMID:
12361163.

27. Lindsay R, Smith G, Atchison D. Descriptors of corneal shape. Optom Vis Sci. 1998; 75:156–158. PMID:
9503441.

28. Gatinel D, Haouat M, Hoang-Xuan T. A review of mathematical descriptors of corneal asphericity. J Fr Ophtalmol. 2002; 25:81–90. PMID:
11965125.
Go to :











 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download