This article has been
cited by other articles in ScienceCentral.
Abstract
Purpose
To evaluate the prognostic factors and outcomes of dexamethasone intravitreal implant (DEX implant) for intravitreal bevacizumab refractory macular edema secondary to branch retinal vein occlusion (BRVO).
Methods
This was a retrospective, interventional case series. Medical records were reviewed, and a total of 38 eyes that were treated with DEX implant for macular edema secondary to BRVO that did not respond to at least two consecutive intravitreal bevacizumab injections (IBIs) were included. Best-corrected visual acuity (BCVA), central subfield macular thickness, and central subfoveal choroidal thickness were evaluated at baseline, 2 months, and 6 months after DEX implantation.
Results
Patients had undergone an average of 6.32 ± 4.66 prior IBI treatments. The average BCVA improved from 0.53 ± 0.26 to 0.41 ± 0.25 and 0.44 ± 0.23 logarithm of the minimal angle of resolution (logMAR) at 2 and 6 months, respectively (p < 0.001). The average central subfield macular thickness was 504.00 ± 121.54 µm at baseline and changed to 293.21 ± 74.17 µm and 427.28 ± 119.57 µm at 2 and 6 months, respectively (p < 0.001 and p = 0.002). Average central subfoveal choroidal thickness was 237.46 ± 92.21 µm at baseline and changed to 204.75 ± 74.74 µm and 226.86 ± 90.77 µm at 2 and 6 months, respectively (p < 0.001 and p = 0.455). Twenty-two eyes (58%) gained ≥0.1 logMAR at 2 months, while 16 eyes showed no improvement. Low BCVA at symptom presentation, low baseline BCVA, and shorter duration of macular edema were correlated with increased BCVA after treatment.
Conclusions
The DEX implant improves functional and anatomical outcomes for up to 6 months in about half of the patients treated with IBI refractory macular edema secondary to BRVO, particularly in patients with low initial and baseline BCVA.
Go to :

Keywords: Bevacizumab, Dexamethasone, Intravitreal injections, Macular edema, Retinal vein occlusion
Retinal vein occlusion (RVO) is the second most common vascular retinopathy after diabetic retinopathy [
1], and macular edema is the most frequent cause of visual impairment in patients with branch retinal vein occlusion (BRVO) [
2]. In RVO, macular edema develops in 5% to 15% of eyes over a 1-year period [
3]. Based upon the Branch Vein Occlusion study [
4], only one-third of the eyes with macular edema due to BRVO and visual acuity <20 / 40 improved to better than 20 / 40 acuity during a 3-year follow-up period.
The results of several studies have shown that laser photocoagulation [
5], intravitreal triamcinolone acetonide [
6], intravitreal anti-vascular endothelial growth factor (VEGF) [
78], and dexamethasone intravitreal implants (DEX implants) [
9] can be beneficial for treating macular edema secondary to RVO. To date, administration of the anti-VEGF compounds ranibizumab and af libercept have been reported to significantly improve visual acuity and reduce macular edema with relatively few complications [
10111213]. Although it has not been used as a prescription treatment, another anti-VEGF, bevacizumab, has shown promising results, with improved visual acuity and a decrease in macular thickness [
14]. Because of its low cost and similar effectiveness in other macular diseases [
15], bevacizumab is also widely used for treating macular edema secondary to BRVO.
More recently, a biodegradable dexamethasone intravitreal implant 0.7 mg (Ozurdex; Allergan, Irvine, CA, USA), which is applied with a sustained delivery, has been shown to both reduce the risk of vision loss and increase the speed and incidence of visual improvement in eyes with macular edema secondary to RVO, with effects that were sustained for up to 6 months after a single injection [
916]. Thus, anti-VEGF and steroid implants are currently widely considered as first-line treatments for macular edema secondary to RVO.
However, macular edema may be persistent and recur repeatedly. Previous studies have shown that repeated anti-VEGF treatments are often required to control macular edema, prevent vision loss, and increase the chance of visual improvement [
12]. In many cases of permanent or recurrent macular edema despite repeated anti-VEGF injections, switching to another medication can be considered. However, the results of the DEX implant for intravitreal bevacizumab injections (IBIs) for refractory macular edema secondary to BRVO are not fully known. Therefore, the purpose of this study was to evaluate the efficacy of DEX implants and to investigate positive prognostic factors of DEX implants in treating macular edema secondary to BRVO refractory to IBI.
Materials and Methods
This retrospective study was approved by the institutional review board of Yonsei University, Seoul, South Korea. All study protocols adhered to the tenets of the Declaration of Helsinki. All patient data were collected from the Department of Ophthalmology, Severance Hospital, and Gangnam Severance Hospital.
We reviewed the medical records of 38 eyes of 38 patients who were treated with the DEX implant for intravitreal bevacizumab (1.25 mg/0.05 mL Avastin; Genentech, South San Francisco, CA, USA) for refractory macular edema secondary to BRVO that did not respond after at least two consecutive IBIs between January 2012 and December 2014. All subjects underwent a comprehensive ophthalmologic examination at the time of initial disease presentation and the beginning of the DEX implant treatments, including initial fluorescein fundus angiography, best-corrected visual acuity (BCVA) determinations, dilated fundus examinations, fundus photography, and spectral domain optical coherence tomography (SD-OCT; Spectralis OCT ver. 1.5.12.0, Heidelberg Engineering, Heidelberg, Germany). After the DEX implant, BCVA measurements, tonometry, slit-lamp biomicroscopy, a dilated fundus examination, and OCT were repeated at the 2 month and 6 month follow-up visits. We designated patients who demonstrated an increase of 0.1 or more logarithm of the minimal angle of resolution (logMAR) BCVA as the responsive group, while the other patients were designated as the nonresponsive group at the 6-month follow-up visit. We also investigated subgroups to identify factors that correlated with response to DEX implant.
The inclusion criteria were as follows: (1) initially treated with two or more consecutive IBIs, (2) refractory to IBI, no improvement or worsening visual acuity, <150 µm reduction in central subfield macular thickness (CSMT), and CSMT >300 µm, and (3) followed-up for at least 6 months after DEX implantation. The following were used as exclusion criteria: severe media opacity, previous vitreoretinal surgery, intraocular inflammation, and other disorders that may have inf luenced macular function (e.g., exudative age-related macular degeneration, proliferative diabetic retinopathy, and epiretinal membrane). Patients with a visual acuity worse than 20 / 400 were also excluded.
The CSMT was defined as the mean retinal thickness of the 1 mm center, as described in the Early Treatment Diabetic Retinopathy Study [
17]. Choroidal thickness was measured by enhanced depth imaging OCT, which was performed by positioning the objective lens of the Spectralis OCT scanner close enough in proximity to invert the image, as described in previous reports [
1819]. Central subfoveal choroidal thickness (CSCT), defined as the vertical distance between the hyperreflective line of Bruch's membrane and the outermost hyperreflective line of the chorioscleral interface at the fovea, was measured manually using the built-in caliber. We used data from horizontal line scans. If it was difficult to identify the outer choroid in its entirety, we chose 10 points at which the chorioscleral interface could be identified easily and created a segmentation line. All image measurements were averaged and were performed by two independent observers (KHL and ECK) who were masked to the clinical information. The visual acuity measurements were converted to the logMAR for analyses.
Statistical analysis
Serial comparisons of the mean BCVA, CSMT, and CSCT were performed using paired t-test. Comparisons of the mean BCVA, CSMT, and, CSCT between the two subgroups (responsive and nonresponsive) were analyzed using the Wilcoxon signed-rank test. Pearson's test was used to identify factors that correlated with visual gain. The statistical significance level was set as p < 0.05. All statistical analyses were performed using SPSS ver. 20.0 for Windows (IBM Corp., Armonk, NY, USA). The p-values less than 0.05 were considered significant.
Go to :

Results
Thirty-eight eyes from 38 patients were included in this study, which included 12 men and 26 women. The mean age was 67.76 ± 10.27 years. Twenty-five eyes (66%) were phakic at the time of the DEX implant. The duration of macular edema between initial presentation and DEX implant was 22.45 ± 19.53 months. The number of previous anti-VEGF injections was 6.32 ± 4.66. The initial mean BCVA at the time of disease presentation was 0.66 ± 0.40 logMAR, and the baseline mean BCVA was 0.53 ± 0.26 logMAR at DEX implantation. The baseline CSMT and CSCT were 504.00 ± 121.54 µm and 237.46 ± 92.21 µm, respectively. Previous grid and sector pan retinal photocoagulation were performed on 3 (8%) and 7 (18%) eyes, respectively (
Table 1).
Table 1
Patient characteristics

Comparison of BCVA, CSMT, and CSCT before and after DEX implantation
Mean logMAR BCVA at 2 months after DEX implantation significantly improved from 0.53 ± 0.26 to 0.41 ± 0.25 (
p < 0.01). The amount of visual gain decreased at 6 months to 0.45 ± 0.23 logMAR, but it was still statistically significant compared to baseline (
p < 0.01). Both CSMT and CSCT were decreased at 2 months after DEX implantation from 504.00 ± 121.54 µm and 237.46 ± 92.21 µm to 293.21 ± 74.17 µm and 204.75 ± 74.74 µm, respectively (both
p < 0.001). Six months after DEX implantation, CSMT and CSCT were slightly increased to 427.28 ± 119.57 µm and 226.86 ± 90.77 µm, respectively. CSMT was still significantly thinner than baseline values (
p = 0.002), but CSCT was not (
p = 0.455) (
Fig. 1A and 1B). Low initial (
p = 0.025) and baseline logMAR BCVA values (
p = 0.028) were correlated with visual gain 2 months after DEX implantation, but age, number of IBIs, initial CSMT, initial CSCT, and thickness change in CSMT and CSCT were not significantly associated with visual change.
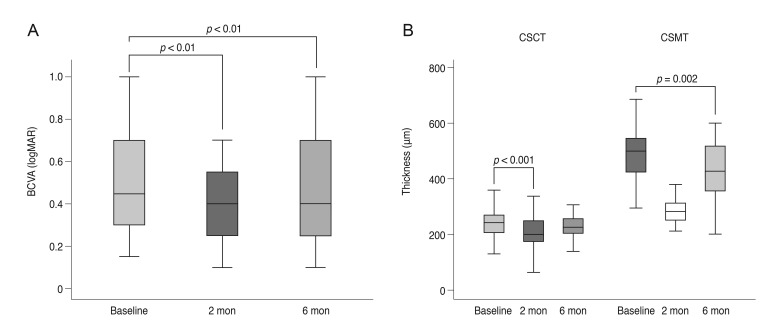 | Fig. 1Functional and anatomical outcomes of dexamethasone intravitreal implants. (A) BCVA change. (B) CSCT and CSMT change. BCVA = best-corrected visual acuity; logMAR = logarithm of the minimal angle of resolution; CSCT = central subfoveal choroidal thickness; CSMT = central subfield macular thickness.
|
Comparison between the responsive and nonresponsive groups
Twenty-two eyes (58%) showed 0.1 or more logMAR BCVA improvement, while 16 eyes (42%) showed no change in BCVA or worsening BCVA. We divided the patients into a responsive group and a nonresponsive group according to this criterion. In the responsive group, the mean initial and baseline logMAR BCVA were significantly higher than those in the nonresponsive group, 0.77 ± 0.46 and 0.61 ± 0.24 compared to 0.49 ± 0.25 and 0.42 ± 0.23 (
p = 0.049 and
p = 0.019), respectively. The duration of macular edema between symptom presentation and DEX implantation for the responsive group was shorter than that of the nonresponsive group, 18.55 ± 17.73 months for the responsive group and 27.81 ± 16.90 months for the nonresponsive group (
p = 0.022). The number of IBIs before DEX implantation was smaller in the responsive group (5.95 ± 5.46 times) than in the nonresponsive group (6.81 ± 3.35 times), but the difference was not statistically significant (
p = 0.195). Age, sex, and previous laser treatment (grid laser or sector pan retinal photocoagulation) were not significantly different between the two groups (
Table 2).
Table 2
Responsive and nonresponsive group comparisons
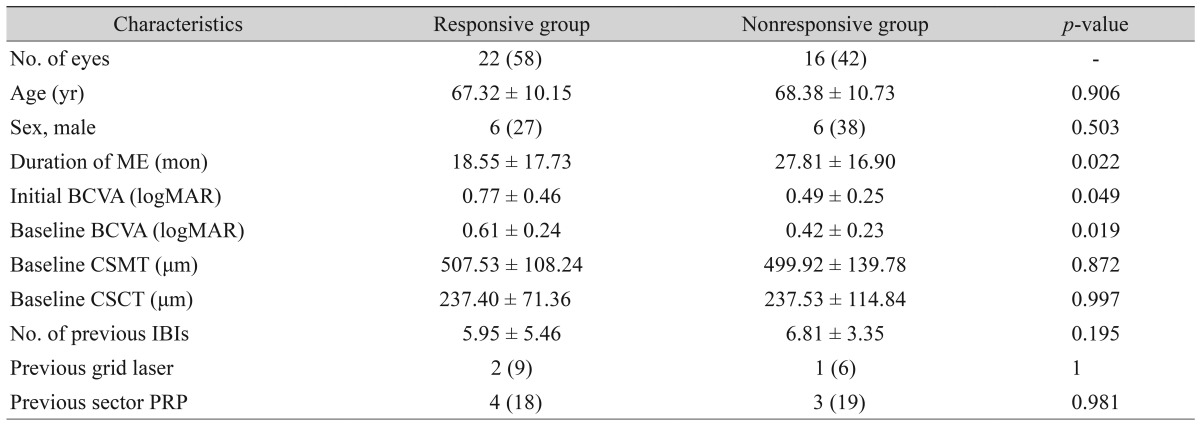

CSMT and CSCT significantly improved 2 months after DEX implantation in both the responsive and nonresponsive groups, but the amount of improvement decreased after 6 months. The mean CSMT changes were 220.40 ± 125.86 µm and 199.69 ± 153.70 µm (p = 0.012 and p = 0.101), respectively, and the mean CSCT changes were 41.69 ± 30.49 µm and 24.93 ± 24.41 µm (p = 0.005 and p = 0.552).
Adverse events
A notable increase in IOP (>10 mmHg from baseline) was noted in five eyes (13.1%), which were subsequently treated with topical antiglaucoma medication. No other complications, including endophthalmitis and retinal detachment, were observed. Two cases showed notable cataract progression and underwent cataract extraction 6 and 8 months after DEX implantation. After a single DEX implant, two eyes (5.26%) needed no additional treatment due to complete resolution of macular edema, which was confirmed by OCT, while the other eyes needed additional treatment such as IBI or the DEX implant.
Go to :

Discussion
In the present study, a single DEX implant was associated with significant improvement of visual acuity in IBI refractory macular edema attributable to BRVO. The mean BCVA improved to 0.12 logMAR at 2 months and 0.08 logMAR at 6 months after the DEX implant, but the efficacy was lower than previous studies that used a DEX implant as a first treatment [
9162021]. However, considering that our patients had long-standing disease with multiple IBIs or laser and clinical IBI refractory macular edema, there was still significant efficacy.
Improvement in BCVA and anatomical change in macular edema likely resulted from the specific effect of dexamethasone, which has somewhat different effects from anti-VEGF agents. The strong efficacy of anti-VEGF treatment indicates that VEGF plays an important role in the development of macular edema due to RVO. However, various cytokines, including interleukin-6 [
2223] and interleukin-8 [
24], can also be an effective treatment for macular edema in RVO. It is possible that the cases of macular edema in our study population refractory to intravitreal anti-VEGF injection involved pathological changes unrelated to VEGF. Furthermore, steroid injection can reduce interleukin-6 [
2526] and interleukin-8 [
26], levels, which cannot be modulated by anti-VEGF therapy.
The CSMT and CSCT of all patients improved after DEX implantation. However, only about half of the patients (22 eyes, 58%) experienced a BCVA improvement greater than 0.1 logMAR. We separated patients into the nonresponsive group and responsive group according to visual gain of 0.1 or more logMAR BCVA. In the subgroup analysis, low BCVA at the time of DEX implant, low BCVA at initial disease presentation, and shorter duration of macular edema were associated with responsiveness to the DEX implant. A small number of patients with previous IBIs also showed an association with DEX implants, but this correlation was not statistically significant (p = 0.195).
Recently, a study reported that CSCT in eyes with RVO was significantly greater than in normal contralateral eyes, and that CSCT decreased significantly after DEX implants [
27]. That study also reported that improved visual acuity correlated with a decrease in CSCT after the DEX implant. These findings are consistent with our data showing that the DEX implant decreased CSCT, but we did not find any significant correlation between CSCT or CSMT and visual improvement.
In our study, the safety profile was consistent with the results of the phase III Geneva Clinical Trial [
916], showing no serious ocular or systemic adverse events during the follow-up period. The most frequent adverse event was an increase in IOP, which required a topical IOP-lowering medication; however, this was only necessary in a small number of patients. Previous studies have reported intravitreal use of steroids for refractory macular edema secondary to RVO [
2829]. To the best of our knowledge, this is the first study to investigate the prognostic factors of DEX implant for bevacizumab refractory macular edema secondary to BRVO. However, there were some limitations to this study, specifically in the retrospective design. The number of anti-VEGF injections before DEX implantation was also not well controlled.
In conclusion, DEX implants were found to be beneficial for patients with IBI refractory macular edema secondary to BRVO. At 2 months after implantation, patients gained a mean 0.12 logMAR BCVA and demonstrated a marked reduction in macular edema that lasted for up to 6 months. Moreover, about half of the patients showed a greater than 0.1 logMAR BCVA gain, with a mean of 0.20 ± 0.13 logMAR BCVA improvement, especially in patients with low initial baseline BCVA and a shorter duration of macular edema. In addition to a relatively convenient dosing schedule, longer drug action period, and low cost with minimal complications, our study suggests that DEX implants can be a valid treatment for IBI refractory macular edema secondary to BRVO. Further studies that involve long-term results of DEX implants for IBI refractory macular edema, together with comparisons of other treatment options with large populations, are therefore warranted to determine optimal treatment approaches.
Go to :

Notes
Go to :

Acknowledgements
Hyoung Jun Koh was a consultant/advisor for Allergan, Bayer, and Novartis Pharmaceuticals Corporation. The funding organizations had no role in the design or conduct of this study.
Go to :

References
1. Mitchell P, Smith W, Chang A. Prevalence and associations of retinal vein occlusion in Australia: the Blue Mountains Eye Study. Arch Ophthalmol. 1996; 114:1243–1247. PMID:
8859084.
2. Campochiaro PA, Hafiz G, Shah SM, et al. Ranibizumab for macular edema due to retinal vein occlusions: implication of VEGF as a critical stimulator. Mol Ther. 2008; 16:791–799. PMID:
18362932.

3. Rogers SL, McIntosh RL, Lim L, et al. Natural history of branch retinal vein occlusion: an evidence-based systematic review. Ophthalmology. 2010; 117:1094–1101.e5. PMID:
20430447.

4. Shilling JS, Jones CA. Retinal branch vein occlusion: a study of argon laser photocoagulation in the treatment of macular oedema. Br J Ophthalmol. 1984; 68:196–198. PMID:
6365157.

5. Scott IU, Ip MS, VanVeldhuisen PC, et al. A randomized trial comparing the efficacy and safety of intravitreal triamcinolone with standard care to treat vision loss associated with macular edema secondary to branch retinal vein occlusion: the Standard Care vs Corticosteroid for Retinal Vein Occlusion (SCORE) study report 6. Arch Ophthalmol. 2009; 127:1115–1128. PMID:
19752420.
6. Ip MS, Scott IU, VanVeldhuisen PC, et al. A randomized trial comparing the efficacy and safety of intravitreal triamcinolone with observation to treat vision loss associated with macular edema secondary to central retinal vein occlusion: the Standard Care vs Corticosteroid for Retinal Vein Occlusion (SCORE) study report 5. Arch Ophthalmol. 2009; 127:1101–1114. PMID:
19752419.
7. Campochiaro PA, Heier JS, Feiner L, et al. Ranibizumab for macular edema following branch retinal vein occlusion: six-month primary end point results of a phase III study. Ophthalmology. 2010; 117:1102–1112.e1. PMID:
20398941.
8. Varma R, Bressler NM, Suner I, et al. Improved vision-related function after ranibizumab for macular edema after retinal vein occlusion: results from the BRAVO and CRUISE trials. Ophthalmology. 2012; 119:2108–2118. PMID:
22817833.
9. Haller JA, Bandello F, Belfort R Jr, et al. Randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with macular edema due to retinal vein occlusion. Ophthalmology. 2010; 117:1134–1146.e3. PMID:
20417567.

10. Brown DM, Campochiaro PA, Bhisitkul RB, et al. Sustained benefits from ranibizumab for macular edema following branch retinal vein occlusion: 12-month outcomes of a phase III study. Ophthalmology. 2011; 118:1594–1602. PMID:
21684606.

11. Campochiaro PA, Brown DM, Awh CC, et al. Sustained benefits from ranibizumab for macular edema following central retinal vein occlusion: twelve-month outcomes of a phase III study. Ophthalmology. 2011; 118:2041–2049. PMID:
21715011.

12. Campochiaro PA, Sophie R, Pearlman J, et al. Long-term outcomes in patients with retinal vein occlusion treated with ranibizumab: the RETAIN study. Ophthalmology. 2014; 121:209–219. PMID:
24112944.
13. Clark WL, Boyer DS, Heier JS, et al. Intravitreal aflibercept for macular edema following branch retinal vein occlusion: 52-week results of the VIBRANT study. Ophthalmology. 2016; 123:330–336. PMID:
26522708.
14. Prager F, Michels S, Kriechbaum K, et al. Intravitreal bevacizumab (Avastin) for macular oedema secondary to retinal vein occlusion: 12-month results of a prospective clinical trial. Br J Ophthalmol. 2009; 93:452–456. PMID:
19074916.
15. Comparison of Age-related Macular Degeneration Treatments Trials (CATT) Research Group. Martin DF, Maguire MG, et al. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmology. 2012; 119:1388–1398. PMID:
22555112.
16. Haller JA, Bandello F, Belfort R Jr, et al. Dexamethasone intravitreal implant in patients with macular edema related to branch or central retinal vein occlusion twelve-month study results. Ophthalmology. 2011; 118:2453–2460. PMID:
21764136.
17. Early Treatment Diabetic Retinopathy Study design and baseline patient characteristics: ETDRS report number 7. Ophthalmology. 1991; 98(5 Suppl):741–756. PMID:
2062510.
18. Chung SE, Kang SW, Lee JH, Kim YT. Choroidal thickness in polypoidal choroidal vasculopathy and exudative age-related macular degeneration. Ophthalmology. 2011; 118:840–845. PMID:
21211846.

19. Margolis R, Spaide RF. A pilot study of enhanced depth imaging optical coherence tomography of the choroid in normal eyes. Am J Ophthalmol. 2009; 147:811–815. PMID:
19232559.

20. Joshi L, Yaganti S, Gemenetzi M, et al. Dexamethasone implants in retinal vein occlusion: 12-month clinical effectiveness using repeat injections as-needed. Br J Ophthalmol. 2013; 97:1040–1044. PMID:
23686324.

21. Capone A Jr, Singer MA, Dodwell DG, et al. Efficacy and safety of two or more dexamethasone intravitreal implant injections for treatment of macular edema related to retinal vein occlusion (Shasta study). Retina. 2014; 34:342–351. PMID:
23846381.

22. Noma H, Funatsu H, Yamasaki M, et al. Aqueous humour levels of cytokines are correlated to vitreous levels and severity of macular oedema in branch retinal vein occlusion. Eye (Lond). 2008; 22:42–48. PMID:
16826241.

23. Noma H, Funatsu H, Mimura T, et al. Vitreous levels of interleukin-6 and vascular endothelial growth factor in macular edema with central retinal vein occlusion. Ophthalmology. 2009; 116:87–93. PMID:
19118700.

24. Fonollosa A, Garcia-Arumi J, Santos E, et al. Vitreous levels of interleukine-8 and monocyte chemoattractant protein-1 in macular oedema with branch retinal vein occlusion. Eye (Lond). 2010; 24:1284–1290. PMID:
20111061.

25. Sohn HJ, Han DH, Lee DY, Nam DH. Changes in aqueous cytokines after intravitreal triamcinolone versus bevacizumab for macular oedema in branch retinal vein occlusion. Acta Ophthalmol. 2014; 92:e217–e224. PMID:
23889803.

26. Park SP, Ahn JK. Changes of aqueous vascular endothelial growth factor and interleukin-6 after intravitreal triamcinolone for branch retinal vein occlusion. Clin Exp Ophthalmol. 2008; 36:831–835. PMID:
19278477.

27. Lee EK, Han JM, Hyon JY, Yu HG. Changes in choroidal thickness after intravitreal dexamethasone implant injection in retinal vein occlusion. Br J Ophthalmol. 2015; 99:1543–1549. PMID:
25883084.

28. Alshahrani ST, Dolz-Marco R, Gallego-Pinazo R, et al. Intravitreal dexamethasone implant for the treatment of refractory macular edema in retinal vascular diseases: results of the KKESH International Collaborative Retina Study Group. Retina. 2016; 36:131–136. PMID:
26079477.
29. Yoo SG, Kim JH, Lee TG, et al. Short-term efficacy of intravitreal triamcinolone acetonide for macular edema secondary to retinal vein occlusion that is refractory to intravitreal bevacizumab. Indian J Ophthalmol. 2015; 63:25–29. PMID:
25686058.

Go to :








 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download