Abstract
Purpose
To investigate the production of long pentraxin 3 (PTX3) in response to tunicamycin-induced endoplasmic reticulum (ER) stress and its role in ER stress-associated cell death, PTX3 expression was evaluated in the human retinal pigment epithelial cell line, ARPE-19.
Methods
PTX3 production in ARPE-19 cells was analyzed in the absence or presence of tunicamycin treatment by enzyme-linked immunosorbent assay. PTX3 protein and mRNA levels were estimated using western blot analysis and real-time reverse transcription-polymerase chain reaction, respectively. Protein and mRNA levels of CCAAT-enhancer-binding protein homologous protein (CHOP) and ARPE-19 cell viability were measured in the presence of tunicamycin-induced ER stress in control or PTX3 small hairpin RNA (shRNA)-transfected ARPE-19 cells.
Results
The protein and mRNA levels of PTX3 were found to be significantly increased by tunicamycin treatment. PTX3 production was significantly decreased in inositol-requiring enzyme 1α shRNA-transfected ARPE-19 cells compared to control shRNA-transfected cells. Furthermore, pretreatment with the NF-κB inhibitor abolished tunicamycin-induced PTX3 production. Decreased cell viability and prolonged protein and mRNA expression of CHOP were observed under tunicamycin-induced ER stress in PTX3 shRNA transfected ARPE-19 cells.
Conclusions
These results suggest that PTX3 production increased in the presence of tunicamycin-induced ER stress. Therefore, PTX3 could be an important protector of ER stress-induced cell death in human retinal pigment epithelial cells. Inositol-requiring enzyme 1α and the NF-κB signaling pathway may serve as potential targets for regulation of PTX3 expression in the retina. Therefore, their role in PTX3 expression needs to be further investigated.
Pentraxins are a superfamily of conserved proteins that are characterized by a cyclic multimeric structure and a conserved C-terminal domain. Classic pentraxins such as C-reactive protein and serum amyloid P are acute-phase proteins made in the liver, that are rapidly activated in response to inflammation [1]. Pentraxin 3 (PTX3, also called TNF-stimulated gene) is the prototypic long pentraxin. It is rapidly produced and released by several cell types, including endothelial cells, fibroblasts, retinal pigment epithelial cells, and particularly mononuclear phagocytes in response to either inflammatory or atheroprotective signals [2345].
PTX3 levels are very low in the serum and tissues of normal subjects; however, its expression rapidly increases in response to inflammatory stimulation in a wide range of diseases, including infectious, autoimmune, and degenerative disorders [678]. Specifically, PTX3 acts as a soluble pathogen recognition receptor with an essential role in resistance against selected pathogens [589]. Several studies have demonstrated that PTX3 is involved in the removal of apoptotic cells during immune response [101112]. PTX3 siRNA knockdown was also shown to promoted cell death, characterized as apoptosis and necrosis, in the presence of proinflammatory cytokines such as IL-1β and TNF-α in conjunctivochalasis fibroblasts [13].
The endoplasmic reticulum (ER) is a key site in the cell for protein folding and trafficking and is central to many cellular functions. Failure of the homeostatic capacity of the ER results in activation of the unfolded protein response (UPR), which leads to the elevated expression of ER chaperones and genes involved in ER expansion, as well as molecules affecting ER and cellular functions. Activation of the UPR generally reflects a loss of ER homeostasis, a condition referred to as ER stress. ER stress plays a fundamental role in the pathogenesis of several diseases such as diabetes, cancer, and neurodegenerative diseases. In ophthalmology, diseases such as glaucoma, diabetic retinopathy, and age-related macular degeneration (ARMD) display features characteristic of ER stress.
In higher eukaryotes, UPR signaling is initiated by three ER transmembrane sensors: inositol-requiring enzyme 1α (IRE1α), pancreatic ER kinase-like ER kinase (PERK), and activating transcription factor 6 [1415]. If proper protein folding capacity in the ER cannot be restored, the UPR upregulates genes such as CCAAT-enhancer-binding protein homologous protein (CHOP), which activates apoptotic pathways [16].
ARMD is a multi-factorial disease and a leading cause of visual impairment in the elderly. Recent studies suggest that production of reactive oxygen species and chronic oxidative stress may play a pivotal role in the development of this disease. The retina, especially the retinal pigment epithelium (RPE), is exposed to high levels of oxidative and photo-oxidative damage over a lifetime. Oxidative stress can inactivate chaperones, promote aberrant disulfide bond formation, promote stabilization of undesirable intermediates, and inhibit degradation of misfolded proteins, which together cause ER stress. However, recent genetic studies suggest a role in the immune response, particularly abnormalities in the innate immune system, in the pathogenesis and severity of ARMD.
The RPE plays an important role in establishing the immune privilege of the eye by secreting immunosuppressive factors [1718]. With these complex and varied roles, the RPE is essential for visual function. Failure in any one of its functions can lead to degeneration of the retina, loss of visual function, and blindness. The RPE, which is the major target cell of ARMD, may be a site for crosstalk between cellular oxidative ER stress and immune signaling. PTX3 produced in RPE cells may play an important protective role in retinal injury against proapoptotic stimuli such as ER stress. In this study, we examined whether PTX3 is regulated by tunicamycin-induced ER stress. We also investigated the role of PTX3 in ER stress-associated cell death in the human retinal pigment epithelial cell line, ARPE-19.
ARPE-19 cells (Fig. 1A and 1B), a human retinal pigment epithelial cell line, were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA). They were cultured in T-75 flasks with Dulbecco's modified Eagle's medium (Invitrogen, Gibco, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (Sigma-Aldrich, St. Louis, MO, USA) and 100 U/mL penicillin and streptomycin (Gibco-BRL, Gaithersburg, MD, USA). During incubation, the culture medium was changed every 2 days. All cultures were maintained at 37℃ under 5% CO2 with 95% relative humidity.
Protein kinase inhibitors such as NF-κB inhibitor (Bay 11-7085) and a c-Jun NH2-terminal kinase (JNK) MAP kinase inhibitor (SP600125; Enzo Life Sciences, Montgomery County, PA, USA), human TNF-α (R&D Systems, Minneapolis, MN, USA), human IL-1β (Abcam, Cambridge, MA, USA), and human IFN-γ (PeproTech, Rocky Hill, NJ, USA) were used. Human PTX3 monoclonal antibody (Novus Biologicals, Littleton, CO, USA) and β-actin polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) were used for western blot analysis. All signaling antibodies were purchased from Cell Signaling Technology (Danvers, MA, USA).
Total RNA from ARPE-19 cells was isolated using a reagent (Invitrogen), and reverse transcription was performed using Moloney murine leukemia virus reverse transcriptase (Invitrogen). The amplification of cDNA by polymerase chain reaction was performed using human PTX3-specific primers and the AccuPower PCR PreMix kit (Bioneer, Daejeon, Korea). β-actin mRNA expression served as a control. Primers were designed using the Primer Express 1.5 software (Applied Biosystems, Carlsbad, CA, USA) and synthesized by Bioneer. The primer sequences were as follows: human PTX3 forward primer, 5′-AATGCATCTCCTTGCGATTC-3′; reverse primer, 5′-TGAAGTGCTTGTCCCATTCC-3′; IRE1α forward primer, 5′-CACAGTGAC GCTTCCTGAAAC-3′; reverse primer, 5′-GCCATCATTAGGATCTGGGAGA-3′; CHOP forward primer, 5′-CCGTTTCCTGGTTCTCCCTTGG-3′; reverse primer, 5′-TGCTTCTCT GGCTTGGCTGAC-3′, and β-actin forward primer, 5′-ATGGTGCGTGACATTAAGGAGAAG-3′; reverse primer, 5′-AGGAAGGAAGGCTGGAAGAGTG-3′. Amplification of cDNA started with 10 minutes at 95℃, followed by 40 cycles of 15 seconds at 95℃ and 1 minute at 59℃. Reverse transcription-polymerase chain reaction for PTX3 ; IRE1α, CHOP, and β-actin was conducted using iQ SYBR Green Supermix (Bio-Rad, Hercules, CA, USA).
Western blot analyses were performed as previously described [19]. Briefly, the cells were harvested using RIPA buffer (Sigma-Aldrich) containing protease inhibitors (Roche Applied Science, Mannheim, Germany). Protein concentrations were determined using the Pierce BCA protein assay kit (Thermo Scientific, Rockford, IL, USA). Samples were resolved by 12% SDS-PAGE gels and transferred to PVDF membranes (Bio-Rad) overnight (120 mA). The transferred membranes were hybridized with various primary antibodies (diluted 1 : 1000, Cell Signaling Technology) overnight at 4℃, followed by incubation with secondary HRP-conjugated IgG antibodies and visualized with SuperSignal West Pico Chemiluminescent Substrate (Thermo Scientific).
Human PTX3 released into the culture medium was measured using an enzyme-linked immunosorbent assay (ELISA) kit (human PTX3/TSG-14) from R&D Systems, according to the manufacturer's instructions. Briefly, ELISA plates (BD Biosciences, San Jose, CA, USA) were coated with a monoclonal anti-human PTX3 antibody (2 µg/mL) in coating buffer (1% BSA in PBS, pH 7.2 to 7.4) overnight at room temperature. The plates were then blocked with coating buffer for 2 hours at room temperature and incubated with either recombinant human PTX3 standards or samples collected in quadruplicate (100 µL/well) for another 2 hours. The plates were then incubated with a biotinylated human PTX3 antibody (150 ng/mL) for 2 hours and subsequently incubated with freshly diluted streptavidin-HRP for 20 minutes in the dark. After each step, the plates were washed three times with washing buffer. A chromogenic substrate tetramethylbenzidine (100 µL/well) (eBioscience, San Diego, CA, USA) was added and incubated for 5 minutes in the dark. The reaction was stopped by adding 2 N H2SO4 (50 µL/well), and the plates were read at 450 nm using an automatic ELISA reader (Merck Sensident Scan, Helsinki, Finland)
Cell viability was determined by the MTS assay using the CellTiter 96 AQueous one solution cell proliferation assay kit (Promega, Madison, WI, USA). Cells were seeded at 0.7 × 104 cells per well in 96-well plates. After reagent treatment, 20 µL of MTS solution was added to each well, and plates were incubated for an additional 2 to 4 hours at 37℃. Absorbance was then measured at 490 nm using a SpectraMax M2 microplate reader (Molecular Devices, Sunnyvale, CA, USA) to calculate cell survival percentages.
Cells were seeded on 12-well plates containing glass coverslips and treated the following day with tunicamycin (1 µM) for 48 hours. Nuclear counterstaining was performed by staining with a solution of 1 µg/mL Hoechst 33258 for 5 minutes and mounting on a slide using fluorescence mounting medium (Dako, Glostrup, Denmark). Cell viability was assessed using a fluorescence microscope.
ARPE-19 cells stably transfected with the pMSCV-LMP retro-puro vector carrying specific small hairpin RNA (shRNA) targeting nucleotides to PTX3 (PTX3 shRNA) or IRE1α (IRE1α shRNA) were generated according to the manufacturer's instructions (OPEN Biosystems, Lafayette, CO, USA). Briefly, the PTX3 shRNA oligo (5′-TGCTGTTGACAGTGAGCGCCCATCAACCTCTCTTCTGTATAGTGAAGCCACAGATGTATACAGAAGAGAGGTTGATGGGTGCCTACTGCCTCGGA-3′) and IRE1α shRNA oligo (5′-TGCTGTTGACAGTGAGCGCAGTGCATAATAGGAACACTTTAGTGAAGCCACAGATGTAAAGTGTTCCTATTATGCACTGTGCCTACTGCCTCGGA-3′) were synthesized and amplified using miR30 common primers (forward primer: 5′-CAGAAGGCTCGAGAAGGTATATTGCTGTTGACAGTGAGCG-3′, reverse primer: 5′-CTAAAGTAGCCCCTTGAATTCCGAGGCAGTAGGCA-3′). The polymerase chain reaction products were digested with EcoRI and XhoI and ligated into pMSCV-LMP retro-puro (OPEN Biosystems). Transfected cells were selected in media containing 2 µg/mL puromycin (Life technologies, Carlsbad, CA, USA).
Data are represented as mean ± standard deviation. For comparisons between two groups, we used the Student's two-tailed unpaired t-test. For comparisons of timed series experiments, we performed Student's paired t-tests. Statistically significant differences were designated at p < 0.05.
ER stress is linked to several pathological conditions, including ARMD [202122]. To verify the cellular effects of the ER stress inducer, tunicamycin, ARPE-19 cells were exposed to 1 µg/mL tunicamycin or the vehicle control (1% DMSO) for 48 hours. The cells were harvested and nuclei stained using Hoechst 33342 (Fig. 2A). Live cells were counted and are represented as a graph in Fig. 2B and 2C. Tunicamycin-induced ER stress accelerated the death of ARPE-19 cells. To verify these results, we assessed ER stress-induced cell death in the absence or presence of tunicamycin using the CellTiter AQueous one solution assay kit (Promega, Leiden, The Netherlands). The viability of ARPE-19 cells decreased with 1 µg/mL tunicamycin treatment (24.2%) compared to vehicle (100%). These results indicate that excessive ER stress induces cell death in ARPE-19 cells [2223].
To investigate the effects of ER stress on PTX3 production, PTX3 expression was measured from supernatants after 48-hour treatment with the vehicle or tunicamycin (0.01, 0.1, and 1 µg/mL) (Fig. 3A). Tunicamycin induced PTX3 production in a dose-dependent manner. Furthermore, a time course analysis in which ARPE-19 cells were treated with 1 µg/mL tunicamycin for 6, 12, 24, 36, and 48 hours demonstrated that PTX3 production began to increase by 12 hours, and a more striking increase in PTX3 was evident after 36 hours of tunicamycin treatment (Fig. 3B). PTX3 production by tunicamycin treatment was significantly enhanced by 48 hours. These results suggest that tunicamycin induced ER stress can enhance the production of PTX3 in human retinal pigment epithelial cells in both a dose and time dependent manner.
To ascertain the effect of tunicamycin induced ER stress on PTX3 mRNA levels in ARPE-19 cells, total RNA was isolated 24 hours after treatment with increasing doses of tunicamycin (0.01, 0.1, and 1 µg/mL) and at different time points (3, 6, 12, 24, 36, and 48 hours) using 0.1 µg/mL of tunicamycin (Fig. 4A and 4B). PTX3 mRNA levels increased with increasing tunicamycin concentration (Fig. 4A). Furthermore, PTX3 mRNA began to increase by 6 hours, and a more striking increase was observed after 36 hours of tunicamycin treatment (Fig. 4B). Taken together, these results suggest that tunicamycin-induced ER stress increased PTX3 production at the transcriptional level.
In order to verify which signaling pathway could be responsible for the stimulation of PTX3 production by tunicamycin-induced ER stress, we used a specific NF-κB inhibitor (Bay 11-7085) and a JNK MAP kinase inhibitor (SP600125). ARPE-19 cells were treated with Bay 11-7085 (10 µM) and SP600125 (10 µM) in the presence of tunicamycin, and supernatants were harvested 48 hours after treatment. Bay 11-7085 blocked the stimulation of PTX3 production by tunicamycin (Fig. 5A); however, SP600125 had no effect on PTX3 production in the presence of tunicamycin (Fig. 5A). These data suggest that the NF-κB signaling pathway may facilitate increased PTX3 production during ER stress in human retinal pigment epithelial cells.
In higher eukaryotes, there are three major ER stress sensors, IRE1α, PERK, and activating transcription factor 6 [2425]. These three ER stress response branches control the expression of specific transcription factors and signaling events that modulate a variety of downstream ER stress responses, which orchestrate adaptation to ER stress. In addition to upregulating UPR target genes, mammalian IRE1α signals through additional pathways include NF-κB and JNK [26]. To determine whether IRE1α is involved in the upregulation of PTX3 during ER stress, we generated control and IRE1α shRNA transfected ARPE-19 cells. Real-time reverse transcription-polymerase chain reaction analyses were performed to assess the degree of IRE1α silencing (Fig. 5B). IRE1α, shRNA transfected ARPE-19 cells abrogated tunicamycin-induced PTX3 production (Fig. 5C). These data suggest that IRE1α is an important mediator of PTX3 production by the ER stress inducer, tunicamycin.
To investigate whether PTX3 production altered cell viability during ER stress, we generated control and PTX3 shRNA-transfected ARPE-19 cells. Western blot analyses were performed to assess the degree of PTX3 silencing (Fig. 6A). The cell viability of PTX3 shRNA transfected ARPE-19 cells decreased with tunicamycin treatment (1 µg/mL, 30.8%), as compared to the vehicle-treated control (55.4%) (Fig. 6B). These data suggest that PTX3 promotes cell survival during ER stress induced by tunicamycin in ARPE-19 cells.
During physiological ER stress, transient CHOP expression is beneficial. However, in pathologically chronic ER stress, prolonged CHOP expression promotes cell death, and it has been implicated in a number of diseases, including ARMD, neurodegenerative diseases, atherosclerosis, diabetes, and renal disease [27282930]. To investigate the effects of PTX3 knockdown on CHOP expression, we treated control and PTX3 shRNA transfected ARPE-19 cells with tunicamycin (1 µg/mL) and harvested RNA and protein at 6, 12, 24, and 48 hours after treatment. CHOP mRNA and protein levels increased after 6 hours and declined at 12 hours in the control shRNA transfected ARPE-19 cells (Fig. 7A and 7B, respectively). However, CHOP mRNA and protein levels increased after 6 hours, and they were prolonged until 48 hours (mRNA) or 24 hours (protein) in PTX3 shRNA-transfected ARPE-19 cells (Fig. 7A and 7B, respectively). Fold changes in the signal intensity of protein expression were calculated in comparison to the control in control shRNA transfected cells (black) or PTX3 shRNA transfected cells (white) (Fig. 7C). Taken together, these results indicate that ER stress stimulates PTX3 production via an IRE1α-dependent signaling pathway that promotes cell survival in response to ER stress-induced cell death in ARPE-19 cells.
RPE cell absorb light energy focused on the retina by the lens, and contribute to the maintenance of photoreceptor excitability. Damage to any of the intricate structures of the retina can lead to visual impairment and blindness [3132]. Apoptosis is a common form of cell death observed in RPE cells. It is also an important feature of ARMD, the most common cause of irreversible vision loss, particularly in individuals over 65 years of age [33]. Age-related alterations in the RPE include a reduction in cell density, which can be caused by apoptosis resulting from accumulation of toxic substances [34]. Oxidative stress, hyperglycemia, mitochondrial dysfunction, and ER stress are among the many studied pro-apoptotic factors in RPE cell lines [35363738]. Recent studies suggest that the ER may also play an important role in response to oxidative stress-induced damage in neuronal and epithelial cells [394041]. ER stress has been identified in the tissues of patients with inherited macular degeneration. Therefore, ER stress in RPE could play an important role in the pathogenesis of choroidal neovascularization complicating AMD [4243].
Pentraxins are key components of the humoral arm of innate immunity, which also include the complement system, collectins, and ficolins [1]. PTX3 is the first member of the long pentraxin subfamily and was identified in the early 1990s as a new secreted protein rapidly induced by IL-1β in endothelial cells or by TNF in fibroblasts [4445]. PTX3 plays a non-redundant role in humoral innate immunity, assisting in the resistance to selected pathogens by recognizing microbes, activating complement, and facilitating pathogen recognition by phagocytes. PTX3 is also essential in female fertility [446474849] and acts as a scavenger of cell debris, as well as an immunosuppressant. It specifically binds to late apoptotic cells and subsequently inhibits their uptake by dendritic cells, thus acting as an additional tool for maintaining immune tolerance [1050]. PTX3 binds to complement factor H, a complement regulator protein whose main function is to inhibit activation of the alternative complement pathway. Its polymorphic variant, harboring a substitution of histidine for tyrosine at codon Y420H, has been shown to be strongly associated with ARMD [51].
We previously reported that human retinal pigment epithelial cells are a major source for PTX3 production in the presence of the proinflammatory cytokines IL-1β and TNF-α, and they could be important mediators for host defense and inflammatory response in the retina [52]. We also showed that PTX3 concentration was significantly higher in neovascular ARMD patients and diabetic retinopathy patients than in control patients [5354]. These data suggest that PTX3 produced in RPE cells may play an important role in protection against inflammation and proapoptotic factors, including ER stress, during retinal injury. Furthermore, Wu et al. [55] demonstrated that PTX-3 secretion was regulated during thapsigargin-induced ER stress in cumulus-oocyte complexes.
In this study, we found that a human RPE cell line, ARPE-19, locally produced PTX3, and its expression and production were increased by tunicamycin-induced ER stress via an IRE1α-mediated NF-κB signaling pathway. As shown in Fig. 6, PTX3 shRNA transfected cells were more sensitive to tunicamycin-induced cell death. This was because of prolonged expression of CHOP, a mediator of apoptosis during ER stress (Fig. 7). These data suggest that PTX3 produced in RPE cells play an important protective role in retinal injury against proapoptotic stimuli such as ER stress. The role of PTX3 production in the presence of other pathological stimuli related to retinal degenerative and other eye diseases needs to be investigated.
Figures and Tables
 | Fig. 1(A) ARPE-19 cells, a human retinal pigment epithelial cell line, that show morphologic retinal pigment epithelium (RPE). The image was obtained using an optical microscope at 100× magnification without staining. (B) Real-time polymerase chain reaction analysis at sequential time points for the expression of key genes in RPE differentiation and development. Undifferentiated HT29 cells (human colon carcinoma cell line) were used as a negative control. Expression of paired box protein 6, microphthalmia-associated transcription factor, and orthodenticle homeobox 2 were higher in human retinal pigment epithelium (hRPE) compared to HT29 cells. hPAX6 = human paired box protein 6; hMITF = human microphthalmia-associated transcription factor; hOTX2 = human orthodenticle homeobox 2. |
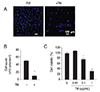 | Fig. 2Tunicamycin (TM)-induced endoplasmic reticulum stress accelerates cell death in the human retinal pigment epithelial cell line, ARPE-19. (A) ARPE-19 cells were treated with vehicle or TM (1 µg/mL). After 48 hours, cell nuclei were stained to detect live cells using Hoechst 33342. (B) Quantification of the amount of live cells. Values are mean ± standard deviation, n = 6. *p < 0.05 vs. vehicle. (C) The viability of ARPE-19 cells was measured 48 hours after vehicle or TM (0.01, 0.1, and 1 µg/mL) treatment using the Ez-Cytox Cell Viability Assay Kit. Values are mean ± standard deviation, n = 12. *p < 0.05 vs. vehicle. |
 | Fig. 3Pentraxin 3 (PTX3) production is enhanced by tunicamycin (TM)-induced endoplasmic reticulum stress in ARPE-19 cells. (A) ARPE-19 cells were stimulated with increasing concentrations of TM (0.01, 0.1, and 1 µg/mL). After 48 hours, supernatants were harvested and assessed for PTX3 production. (B) ARPE-19 cells were incubated for various time-periods in the absence or presence of TM (0.1 µg/mL). Supernatants were harvested and measured for PTX3 production at the indicated time points. Values are mean ± standard deviation, n = 10. *p < 0.05 vs. vehicle. |
 | Fig. 4Pentraxin 3 (PTX3) mRNA expression is increased by tunicamycin (TM)-induced endoplasmic reticulum stress in ARPE-19 cells. (A) Total RNA was extracted from ARPE-19 cells after 24-hour treatment with various doses of TM. (B) ARPE-19 cells were incubated for various time-periods in the absence or presence of TM (0.1 µg/mL). PTX3 mRNA expression levels were assessed by real-time reverse transcription-polymerase chain reaction analysis. Three independent experiments were performed. PTX3 expression values were divided by the expression of the control gene, β-actin, and represented as fold increase relative to the vehicle control. *p < 0.05 vs. vehicle. For the real-time polymerase chain reaction experiments, values are presented as mean ± standard deviation, n = 3. |
 | Fig. 5NF-κB signaling induces pentraxin 3 (PTX3) production. (A) ARPE-19 cells were stimulated with vehicle or tunicamycin (TM, 0.1 or 1 µg/mL) in the absence or presence of NF-B (BAY 11-7085) and c-Jun NH2-terminal kinase (SP600125) signaling inhibitors. After 48 hours, supernatants were harvested and assessed for PTX3 production. Results are shown as means ± standard deviation (SD), n = 12. *p < 0.05 vs. vehicle. (B) ARPE-19 cells were stably transfected with a control (CON) vector or small hairpin RNA (shRNA) for inositol-requiring enzyme 1α (IRE1α). The cells were then exposed to vehicle or TM (0.1 µg/mL) for 48 hours. Quantitative reverse transcription-polymerase chain reaction analyses were performed for IRElα and β-actin (loading CON) to assess the degree of IRElα silencing. Three independent experiments were performed. Results are shown as means ± SD, n = 4. *p < 0.05 vs. CON shRNA. (C) PTX3 production was assessed in CON shRNA or IRE1α shRNA transfected ARPE-19 cells in the absence or presence of TM (0.1 µg/mL). Results are shown as means ± SD, n = 4. *p < 0.05 vs. CON shRNA. |
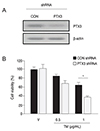 | Fig. 6Cell viability is decreased in pentraxin 3 (PTX3) knock-down ARPE-19 cells. (A) ARPE-19 cells were transfected with a control (CON) vector or with PTX3 small hairpin RNA (shRNA). Western blot analyses for PTX3 expression were also performed to assess the degree of PTX3 silencing. (B) Cell viability of CON shRNA or PTX3 shRNA stable cells was measured 48 hours after vehicle or tunicamycin (TM, 0.5 and 1 µg/mL) treatment using the Ez-Cytox Cell Viability Assay Kit (Itsbio, Seoul, Korea). *p < 0.05: decreased cell viability of PTX3 shRNA cells vs. CON shRNA cells. Values are shown as mean ± standard deviation, n = 12. |
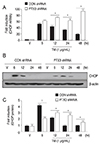 | Fig. 7Tunicamycin (TM)-induced endoplasmic reticulum stress activates prolonged CCAAT-enhancer-binding protein homologous protein (CHOP) expression in pentraxin 3 (PTX3) knockdown ARPE-19 cells. (A) ARPE-19 cells were transfected with a control (CON) vector or with PTX3 small hairpin RNA (shRNA). Total RNA was extracted after various time periods in the absence or presence of tunicamycin (0.1 µg/mL). CHOP mRNA expression was quantified by real-time reverse transcription-polymerase chain reaction analysis, and three independent experiments were performed. The values for CHOP expression were divided by expression of the CON gene, β-actin, and presented as fold increase relative to the vehicle CON. *p < 0.05 increased mRNA levels of CHOP in PTX3 shRNA cells vs. CON shRNA cells. For the real-time polymerase chain reaction experiments, values are presented as mean ± standard deviation (SD), n = 3. (B) CON shRNA and PTX3 shRNA transfected cells were incubated for various time periods in the absence or presence of tunicamycin (0.1 µg/mL). Total protein was harvested and protein levels of CHOP were analyzed by western blot analysis using a human anti-CHOP polyclonal antibody. Results are representative of three independent experiments. (C) The fold change of signal intensity of protein expression was calculated and corrected for loading in vehicle treated cells. *p < 0.05: enhanced CHOP protein levels vs. CON shRNA; †p < 0.05: decreased CHOP protein levels vs. CON shRNA. Values are shown as mean ± SD, n = 3. |
Acknowledgements
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2014R1A6A1030318).
References
1. Goodman AR, Cardozo T, Abagyan R, et al. Long pentraxins: an emerging group of proteins with diverse functions. Cytokine Growth Factor Rev. 1996; 7:191–202.
2. Hamel CP, Detrick B, Hooks JJ. Evaluation of Ia expression in rat ocular tissues following inoculation with interferon-gamma. Exp Eye Res. 1990; 50:173–182.
3. Garlanda C, Bottazzi B, Bastone A, Mantovani A. Pentraxins at the crossroads between innate immunity, inflammation, matrix deposition, and female fertility. Annu Rev Immunol. 2005; 23:337–366.
4. Alles VV, Bottazzi B, Peri G, et al. Inducible expression of PTX3, a new member of the pentraxin family, in human mononuclear phagocytes. Blood. 1994; 84:3483–3493.
5. Doni A, Peri G, Chieppa M, et al. Production of the soluble pattern recognition receptor PTX3 by myeloid, but not plasmacytoid, dendritic cells. Eur J Immunol. 2003; 33:2886–2893.
6. Luchetti MM, Piccinini G, Mantovani A, et al. Expression and production of the long pentraxin PTX3 in rheumatoid arthritis (RA). Clin Exp Immunol. 2000; 119:196–202.
7. Bussolati B, Peri G, Salvidio G, et al. The long pentraxin PTX3 is synthesized in IgA glomerulonephritis and activates mesangial cells. J Immunol. 2003; 170:1466–1472.
8. Garlanda C, Hirsch E, Bozza S, et al. Non-redundant role of the long pentraxin PTX3 in anti-fungal innate immune response. Nature. 2002; 420:182–186.
9. Garlanda C, Maina V, Cotena A, Moalli F. The soluble pattern recognition receptor pentraxin-3 in innate immunity, inflammation and fertility. J Reprod Immunol. 2009; 83:128–133.
10. Rovere P, Peri G, Fazzini F, et al. The long pentraxin PTX3 binds to apoptotic cells and regulates their clearance by antigen-presenting dendritic cells. Blood. 2000; 96:4300–4306.
11. van Rossum AP, Fazzini F, Limburg PC, et al. The prototypic tissue pentraxin PTX3, in contrast to the short pentraxin serum amyloid P, inhibits phagocytosis of late apoptotic neutrophils by macrophages. Arthritis Rheum. 2004; 50:2667–2674.
12. Jaillon S, Jeannin P, Hamon Y, et al. Endogenous PTX3 translocates at the membrane of late apoptotic human neutrophils and is involved in their engulfment by macrophages. Cell Death Differ. 2009; 16:465–474.
13. Guo P, Zhang SZ, He H, et al. PTX3 controls activation of matrix metalloproteinase 1 and apoptosis in conjunctivochalasis fibroblasts. Invest Ophthalmol Vis Sci. 2012; 53:3414–3423.
14. Kaufman RJ. Orchestrating the unfolded protein response in health and disease. J Clin Invest. 2002; 110:1389–1398.
15. Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007; 8:519–529.
16. Okada T, Yoshida H, Akazawa R, et al. Distinct roles of activating transcription factor 6 (ATF6) and double-stranded RNA-activated protein kinase-like endoplasmic reticulum kinase (PERK) in transcription during the mammalian unfolded protein response. Biochem J. 2002; 366:585–594.
17. Ishida K, Panjwani N, Cao Z, Streilein JW. Participation of pigment epithelium in ocular immune privilege. 3. Epithelia cultured from iris, ciliary body, and retina suppress T-cell activation by partially non-overlapping mechanisms. Ocul Immunol Inflamm. 2003; 11:91–105.
18. Streilein JW, Ma N, Wenkel H, et al. Immunobiology and privilege of neuronal retina and pigment epithelium transplants. Vision Res. 2002; 42:487–495.
19. Chung SW, Chen YH, Perrella MA. Role of Ets-2 in the regulation of heme oxygenase-1 by endotoxin. J Biol Chem. 2005; 280:4578–4584.
20. Libby RT, Gould DB. Endoplasmic reticulum stress as a primary pathogenic mechanism leading to age-related macular degeneration. Adv Exp Med Biol. 2010; 664:403–409.
21. Salminen A, Kauppinen A, Hyttinen JM, et al. Endoplasmic reticulum stress in age-related macular degeneration: trigger for neovascularization. Mol Med. 2010; 16:535–542.
22. Jing G, Wang JJ, Zhang SX. ER stress and apoptosis: a new mechanism for retinal cell death. Exp Diabetes Res. 2012; 2012:589589.
23. Zhang SX, Sanders E, Wang JJ. Endoplasmic reticulum stress and inflammation: mechanisms and implications in diabetic retinopathy. J Ocul Biol Dis Infor. 2011; 4:51–61.
24. Gardner BM, Pincus D, Gotthardt K, et al. Endoplasmic reticulum stress sensing in the unfolded protein response. Cold Spring Harb Perspect Biol. 2013; 5:a013169.
25. Maguire JA, Mulugeta S, Beers MF. Multiple ways to die: delineation of the unfolded protein response and apoptosis induced by surfactant protein C BRICHOS mutants. Int J Biochem Cell Biol. 2012; 44:101–112.
26. Hetz C, Martinon F, Rodriguez D, Glimcher LH. The unfolded protein response: integrating stress signals through the stress sensor IRE1α. Physiol Rev. 2011; 91:1219–1243.
27. Li J, Cai X, Xia Q, et al. Involvement of endoplasmic reticulum stress in all-trans-retinal-induced retinal pigment epithelium degeneration. Toxicol Sci. 2015; 143:196–208.
28. Tabas I, Ron D. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat Cell Biol. 2011; 13:184–190.
29. Oyadomari S, Mori M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ. 2004; 11:381–389.
30. Chen C, Cano M, Wang JJ, et al. Role of unfolded protein response dysregulation in oxidative injury of retinal pigment epithelial cells. Antioxid Redox Signal. 2014; 20:2091–2106.
31. Strauss O. The retinal pigment epithelium in visual function. Physiol Rev. 2005; 85:845–881.
32. Bok D. The retinal pigment epithelium: a versatile partner in vision. J Cell Sci Suppl. 1993; 17:189–195.
33. Friedman E. The pathogenesis of age-related macular degeneration. Am J Ophthalmol. 2008; 146:348–349.
34. Hageman GS, Luthert PJ, Victor Chong NH, et al. An integrated hypothesis that considers drusen as biomarkers of immune-mediated processes at the RPE-Bruch’s membrane interface in aging and age-related macular degeneration. Prog Retin Eye Res. 2001; 20:705–732.
35. Zou X, Feng Z, Li Y, et al. Stimulation of GSH synthesis to prevent oxidative stress-induced apoptosis by hydroxytyrosol in human retinal pigment epithelial cells: activation of Nrf2 and JNK-p62/SQSTM1 pathways. J Nutr Biochem. 2012; 23:994–1006.
36. Lim SK, Park MJ, Lim JC, et al. Hyperglycemia induces apoptosis via CB1 activation through the decrease of FAAH 1 in retinal pigment epithelial cells. J Cell Physiol. 2012; 227:569–577.
37. Dou G, Sreekumar PG, Spee C, et al. Deficiency of αB crystallin augments ER stress-induced apoptosis by enhancing mitochondrial dysfunction. Free Radic Biol Med. 2012; 53:1111–1122.
38. Shimazawa M, Inokuchi Y, Ito Y, et al. Involvement of ER stress in retinal cell death. Mol Vis. 2007; 13:578–587.
39. Chen L, Gao X. Neuronal apoptosis induced by endoplasmic reticulum stress. Neurochem Res. 2002; 27:891–898.
40. Paschen W, Mengesdorf T, Althausen S, Hotop S. Peroxidative stress selectively down-regulates the neuronal stress response activated under conditions of endoplasmic reticulum dysfunction. J Neurochem. 2001; 76:1916–1924.
41. Yu Z, Luo H, Fu W, Mattson MP. The endoplasmic reticulum stress-responsive protein GRP78 protects neurons against excitotoxicity and apoptosis: suppression of oxidative stress and stabilization of calcium homeostasis. Exp Neurol. 1999; 155:302–314.
42. Kline CL, Schrufer TL, Jefferson LS, Kimball SR. Glucosamine-induced phosphorylation of the alpha-subunit of eukaryotic initiation factor 2 is mediated by the protein kinase R-like endoplasmic-reticulum associated kinase. Int J Biochem Cell Biol. 2006; 38:1004–1014.
43. Roybal CN, Marmorstein LY, Vander Jagt DL, Abcouwer SF. Aberrant accumulation of fibulin-3 in the endoplasmic reticulum leads to activation of the unfolded protein response and VEGF expression. Invest Ophthalmol Vis Sci. 2005; 46:3973–3979.
44. Breviario F, d'Aniello EM, Golay J, et al. Interleukin-1-inducible genes in endothelial cells: cloning of a new gene related to C-reactive protein and serum amyloid P component. J Biol Chem. 1992; 267:22190–22197.
45. Lee GW, Lee TH, Vilcek J. TSG-14, a tumor necrosis factor-and IL-1-inducible protein, is a novel member of the pentaxin family of acute phase proteins. J Immunol. 1993; 150:1804–1812.
46. Napoleone E, Di Santo A, Bastone A, et al. Long pentraxin PTX3 upregulates tissue factor expression in human endothelial cells: a novel link between vascular inflammation and clotting activation. Arterioscler Thromb Vasc Biol. 2002; 22:782–787.
47. Luchetti MM, Sambo P, Majlingova P, et al. Scleroderma fibroblasts constitutively express the long pentraxin PTX3. Clin Exp Rheumatol. 2004; 22:3 Suppl 33. S66–S72.
48. May L, Kuningas M, van Bodegom D, et al. Genetic variation in pentraxin (PTX) 3 gene associates with PTX3 production and fertility in women. Biol Reprod. 2010; 82:299–304.
49. Bottazzi B, Bastone A, Doni A, et al. The long pentraxin PTX3 as a link among innate immunity, inflammation, and female fertility. J Leukoc Biol. 2006; 79:909–912.
50. Baruah P, Propato A, Dumitriu IE, et al. The pattern recognition receptor PTX3 is recruited at the synapse between dying and dendritic cells, and edits the cross-presentation of self, viral, and tumor antigens. Blood. 2006; 107:151–158.
51. Baruah P, Dumitriu IE, Peri G, et al. The tissue pentraxin PTX3 limits C1q-mediated complement activation and phagocytosis of apoptotic cells by dendritic cells. J Leukoc Biol. 2006; 80:87–95.
52. Woo JM, Kwon MY, Shin DY, et al. Human retinal pigment epithelial cells express the long pentraxin PTX3. Mol Vis. 2013; 19:303–310.
53. Min JK, Kim J, Woo JM. Elevated plasma pentraxin3 levels and its association with neovascular age-related macular degeneration. Ocul Immunol Inflamm. 2015; 23:205–211.
54. Yang HS, Woo JE, Lee SJ, et al. Elevated plasma pentraxin 3 levels are associated with development and progression of diabetic retinopathy in Korean patients with type 2 diabetes mellitus. Invest Ophthalmol Vis Sci. 2014; 55:5989–5997.
55. Wu LL, Russell DL, Norman RJ, Robker RL. Endoplasmic reticulum (ER) stress in cumulus-oocyte complexes impairs pentraxin-3 secretion, mitochondrial membrane potential (DeltaPsi m), and embryo development. Mol Endocrinol. 2012; 26:562–573.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download