1. von Noorden GK. Clinical observations in cyclodeviations. Ophthalmology. 1979; 86:1451–1461.
2. Parks MM. The overacting inferior oblique muscle. Am J Ophthalmol. 1974; 77:787–797.
3. Kushner BJ. The effect of oblique muscle surgery on the axis of astigmatism. J Pediatr Ophthalmol Strabismus. 1986; 23:277–280.
4. Sim JH, Lee SY. The effect of inferior oblique weakening procedures on the correction of ocular torsion. J Korean Ophthalmol Soc. 2005; 46:1020–1026.
5. Hong SW, Kang NY. Astigmatic changes after horizontal rectus muscle surgery in intermittent exotropia. Korean J Ophthalmol. 2012; 26:438–445.
6. Noh JH, Park KH, Lee JY, et al. Changes in refractive error and anterior segment parameters after isolated lateral rectus muscle recession. J AAPOS. 2013; 17:291–295.
7. Emre S, Cankaya C, Demirel S, Doganay S. Comparison of preoperative and postoperative anterior segment measurements with Pentacam in horizontal muscle surgery. Eur J Ophthalmol. 2008; 18:7–12.
8. Mun GH, Heo H, Park SW, Park YG. The changes of corneal astigmatism and refraction after horizontal rectus muscle surgery in intermittent exotropia. J Korean Ophthalmol Soc. 2010; 51:581–587.
9. Snir M, Nissenkorn I, Buckman G, et al. Postoperative refractive changes in children with congenital esotropia: a preliminary study. Ophthalmic Surg. 1989; 20:57–62.
10. Marshall D. Changes in refraction following operation for strabismus. Arch Ophthalmol. 1936; 15:1020–1031.
11. Dottan SA, Hoffman P, Oliver MD. Astigmatism after strabismus surgery. Ophthalmic Surg. 1988; 19:128–129.
12. Preslan MW, Cioffi G, Min YI. Refractive error changes following strabismus surgery. J Pediatr Ophthalmol Strabismus. 1992; 29:300–304.
13. Killer HE, Bahler A. Significant immediate and long-term reduction of astigmatism after lateral rectus recession in divergent Duane's syndrome. Ophthalmologica. 1999; 213:209–210.
14. Nardi M, Rizzo S, Pellegrini G, Lepri A. Effects of strabismus surgery on corneal topography. J Pediatr Ophthalmol Strabismus. 1997; 34:244–246.
15. Kwitko S, Feldon S, McDonnell PJ. Corneal topographic changes following strabismus surgery in Grave's disease. Cornea. 1992; 11:36–40.
16. Thompson WE, Reinecke RD. The changes in refractive status following routine strabismus surgery. J Pediatr Ophthalmol Strabismus. 1980; 17:372–374.
17. Hainsworth DP, Bierly JR, Schmeisser ET, Baker RS. Corneal topographic changes after extraocular muscle surgery. J AAPOS. 1999; 3:80–86.
18. Chun BY, Kim HK, Kwon JY. Comparison of magnitude of astigmatism induced by lateral rectus recession. Optom Vis Sci. 2010; 87:61–65.
19. Retzlaff J, Paden PY, Ferrell L. Vector analysis of astigmatism: adding and subtracting spherocylinders. J Cataract Refract Surg. 1993; 19:393–398.
20. Holladay JT, Moran JR, Kezirian GM. Analysis of aggregate surgically induced refractive change, prediction error, and intraocular astigmatism. J Cataract Refract Surg. 2001; 27:61–79.
21. Bagheri A, Farahi A, Guyton DL. Astigmatism induced by simultaneous recession of both horizontal rectus muscles. J AAPOS. 2003; 7:42–46.
22. Bartier M, Putteman A. Changes in astigmatism following surgery for strabismus. Bull Soc Belge Ophtalmol. 1988; 229:87–96.
23. Santiago AP, Isenberg SJ, Apt L, Roh YB. The effect of anterior transposition of the inferior oblique muscle on ocular torsion. J AAPOS. 1997; 1:191–196.
24. Ruttum M, von Noorden GK. Adaptation to tilting of the visual environment in cyclotropia. Am J Ophthalmol. 1983; 96:229–237.
25. von Noorden GK. Clinical and theoretical aspects of cyclotropia. J Pediatr Ophthalmol Strabismus. 1984; 21:126–132.
26. Al-Haddad C, Antonios R, Khatib L, et al. Is inferior oblique overaction associated with astigmatism? J Pediatr Ophthalmol Strabismus. 2015; 52:288–293.
27. Rajavi Z, Rabei HM, Ramezani A, et al. Refractive effect of the horizontal rectus muscle recession. Int Ophthalmol. 2008; 28:83–88.
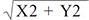 ,
Axis of induced astigmatism = arctan (Y / X) / 2
,
Axis of induced astigmatism = arctan (Y / X) / 2



 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download