Abstract
Purpose
Methods
Results
Figures and Tables
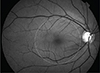 | Fig. 1The central circle (6-mm diameter) corresponds to the ganglion cell inner plexiform layer map size overlapping the redfree retinal nerve fiber layer photograph to determine central retinal nerve fiber layer progression. |
Table 1
Demographic and clinical characteristics of the participants

Values are presented as mean ± standard deviation.
SE = spherical equivalent; IOP = intraocular pressure; CCT = central corneal thickness; VF = visual field; MD = mean deviation; PSD = pattern standard deviation; cRNFL = circumpapillary retinal nerve fiber layer; GC-IPL = ganglion cell inner plexiform layer.
*Test variability assessment group in non-progressed preperimetric glaucomatous eye; †Main analysis group; ‡Comparison between group A and group B by an unpaired t-test and the chi-square test.
Table 2
Longitudinal measurement variability determined by SD-OCT (n = 110)
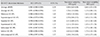
SD-OCT = spectral domain optical coherence tomography; ICC = intraclass correlation coefficient; CI = confidence interval; COV = coefficient of variation; cRNFL = circumpapillary retinal nerve fiber layer; GC-IPL = ganglion cell inner plexiform layer.
*Test-retest variability defined at the 95% confidence level, with the 95% CI shown in parentheses; †Test-retest variability defined at the 80% confidence level, with the 95% CI shown in parentheses.
Table 3
Sensitivity and specificity of average cRNFL thickness and GC-IPL parameters for detection of overall VF progression (n = 274)

cRNFL = circumpapillary retinal nerve fiber layer thickness; GC-IPL = ganglion cell inner plexiform layer; VF = visual field.
*Test-retest variability defined at the 95% confidence level, shown with the 95% confidence interval in parentheses; †Test-retest variability defined at the 80% confidence level, shown with the 95% confidence interval in parentheses.
Table 4
Sensitivity and specificity of average cRNFL thickness and GC-IPL parameters to detect central VF progression (n = 274)

cRNFL = circumpapillary retinal nerve fiber layer thickness; GC-IPL = ganglion cell inner plexiform layer; VF = visual field.
*Test-retest variability defined at the 95% confidence level, shown with the 95% confidence interval in parentheses; †Test-retest variability defined at the 80% confidence level, shown with the 95% confidence interval in parentheses.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download