Abstract
Purpose
Methods
Results
Figures and Tables
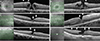 | Fig. 1Preoperative spectral domain-optical coherence tomography images of full-thickness macular hole (A,B), impending macular hole (C,D), and lamellar macular hole (E,F); (A) patient no. 10, (B) patient no. 7, (C) patient no. 8, (D) patient no. 6, (E) patient no. 3, and (F) patient no. 5. Note the thick proliferative tissue mounted at the edge of the hole (bold arrows). Eyes with full-thickness macular hole or impending macular hole were regarded to have inherent disruption of the inner/outer segment junction of the photoreceptor layer. Eyes with lamellar macular hole (E,F) are considered to have defective inner/outer segment junction when an ellipsoidal defect or disconnection is observable (bold triangle). |
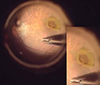 | Fig. 2Perifoveal crown phenomenon observed intraoperatively (patient no. 5). The internal limiting membrane is about to be peeled off with a retinal forceps. The perifoveal crown tissue is then preserved. |
 | Fig. 3Consecutive sections of preoperative macular configuration at a single instance in two patients; patient no. 5 (A,B,C,D) and patient no. 12 (E,F,G,H). Successive sections enable us to identify anatomical continuity of epiretinal proliferation associated with macular hole to the defective base of the lamellar macular hole (outlined manually by the translucent white line). Note that epiretinal proliferation associated with macular hole tissue appears to creep out of a defective outer retina and proliferate beyond the edge of the hole. |
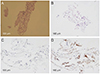 | Fig. 4Histological analysis of perifoveal crown tissue acquired from patient no. 1 (A) and patient no. 3 (B-D). (A) Hematoxylin and eosin stain staining of paraffin-embedded perifoveal crown tissue from patient no. 1. Aggregation of lymphocytes and cells with medium-density nucleus are scattered. (B) Hematoxylin and eosin staining of paraffin-embedded perifoveal crown tissue from patient no. 3. (C) Synaptophysin staining of perifoveal crown tissue from patient no. 3. No specific staining is observed. (D) Pan-keratin staining of perifoveal crown tissue from patient no. 3. Note cells with brownish-stained cytoplasm scattered throughout the specimen. |
Table 1
Demographics of 16 patients with epiretinal proliferation associated with LH or MH

LH = lamellar hole; MH = macular hole; DM = diabetes mellitus; HTN = hypertension; SE = spherical equivalent; VA = visual acuity in logarithm of the minimal angle of resolution; Dx = diagnosis; ICG = indocyanine green; F = female; I-MH = impending macular hole; ERM = epiretinal membrane; DR = diabetic retinopathy; RD = retinal detachment; M = male; C3F8 = perfluoropropane; FT-MH = full-thickness macular hole.
*Patients no. 12 to 16 were followed-up periodically without surgical intervention.
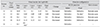
Table 2
Results of initial spectral domain-optical coherence tomography morphological analysis

Values are presented as mean ± standard deviation.
EPMH = epiretinal proliferation associated with macular hole; LH = lamellar hole; FT-MH = full-thickness macular hole; I-MH = impending macular hole.
*Mann-Whitney U-test was performed to compare mean values; †Data in parentheses indicate number of eyes with or without EPMH.
Table 3
Surgical outcomes in relation to visual acuity and macular configuration in patients no. 1 to 11
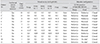
logMAR = logarithm of the minimal angle of resolution; Preop = preoperative; POM = postoperative month; IS/OS = inner/outer segment.
*In patients no. 1 to 3 (shaded rows), the perifoveal crown tissue was removed during the operation; †Patient no. 7 had relatively favorable initial visual acuity despite full thickness macular hole indicated by spectral domain-optical coherence tomography; ‡Follow-up spectral domain-optical coherence tomography image was not available in two patients (no. 10 and 11).




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download