Abstract
Purpose
To investigate the incidence of neovascularization of the iris (NVI) and clinical features of patients with NVI following acute central retinal artery occlusion (CRAO).
Methods
A retrospective review of 214 consecutive CRAO patients who visited one tertiary hospital between January 2009 and January 2015 was conducted. In total, 110 patients were eligible for this study after excluding patients with arteritic CRAO, a lack of follow-up, iatrogenic CRAO secondary to cosmetic filler injection, or NVI detected before CRAO attack. Fluorescein angiography (FA) was applied until retinal arterial reperfusion was achieved, typically within 1 to 3 months.
Results
The incidence of NVI was 10.9% (12 out of 110 patients). Neovascular glaucoma was found in seven patients (6.4%). The mean time to NVI diagnosis after CRAO events was 3.0 months (range, 1 week to 15 months). The cumulative incidence was 5.5% at 3 months, 7.3% at 6 months, and 10.9% at 15 months. Severely narrowed ipsilateral carotid arteries were observed in only three patients (27.3%). The other nine patients (75.0%) showed no predisposing conditions for NVI, such as proliferative diabetic retinopathy or central retinal vein occlusion. Reperfusion rate and prevalence of diabetes were significantly different between patients with NVI and patients without NVI (reperfusion: 0% [NVI] vs. 94.7% [no NVI], p < 0.001; diabetes: 50.0% [NVI] vs. 17.3% [no NVI], p = 0.017).
Conclusions
CRAO may lead to NVI and neovascular glaucoma caused by chronic retinal ischemia from reperfusion failure. Our results indicate that follow-up fluorescein angiography is important to evaluate retinal artery reperfusion after acute CRAO events, and that prophylactic treatment such as panretinal photocoagulation should be considered if retinal arterial perfusion is not recovered.
Central retinal artery occlusion (CRAO) leads to sudden painless vision loss. CRAO is an ophthalmologic emergency, analogous to an acute stroke. The incidence is estimated to be 1.80 in 100,000 people [1]. CRAO can induce neovascularization of the iris (NVI) and neovascular glaucoma (NVG), with a reported incidence of 1% to 20% [2]. Neovascularization (NV) of the iris is a relatively rare complication observed in the setting of retinal ischemic disease, including proliferative diabetic retinopathy (PDR), ocular ischemic syndrome (OIS), or central retinal vein occlusion (CRVO). NVI can progress to the trabecular meshwork, causing narrowing of the aqueous humor outlet and impairing aqueous humor circulation. Eventually, NVG leads to intractable eye pain, and even phthisis bulbi and total blindness. CRAO is an acute retinal ischemic disease [3]. Some researchers argue that CRAO alone cannot induce NVI or NVG, while others have reported a possible association. The link between CRAO and NVI remains unclear. We undertook this study to evaluate the relationship between CRAO and NVI.
We present a case series of patients with CRAO complicated by NVI, and the incidence of NVI in CRAO patients. We conclude that NVI development following a CRAO attack is possible. Furthermore, we compared clinical features between patients with NVI and those without NVI.
This study was approved by the institutional review board of Seoul National University Bundang Hospital. A retrospective review of consecutive CRAO patients from one tertiary hospital between January 2009 and January 2015 was performed. Exclusion criteria included patients with arteritic CRAO, lack of follow-up period, iatrogenic CRAO following cosmetic filler injection, and cases in which CRAO following NVI could not be shown. Additionally, previous reports have shown that NVI following CRAO appears within 4 months [45]. Therefore, patients who did not comply with follow-up within 4 months were excluded.
In total, 214 patients were reviewed and 110 patients met the inclusion criteria (Fig. 1). A total of 12 NVI cases were identified. The diagnosis of CRAO was confirmed by the initial ophthalmologist's clinical diagnosis, with the patient's history of severe sudden visual loss and fundoscopic examination revealing a cherry red spot and retinal whitening in the posterior pole. NVI was defined as an abnormal vessel in the iris as identified through slit lamp microscopic examination, and NVG was defined as increased intraocular pressure (IOP) greater than 21 mmHg, with NVI.
Intra-arterial thrombolysis was performed in selected patients who met the treatment criteria [6] and agreed to the treatment. Other CRAO patients received conservative treatment, including ocular massage, anterior chamber paracentesis, hyperbaric oxygen, and anticoagulants.
After CRAO was diagnosed, ocular examination included a detailed testing of visual acuity with a visual field test using a Goldmann perimeter, a careful anterior segment examination with slit-lamp microscopy, and IOP measurements using a Goldmann applanation tonometer. In addition to these evaluations, fluorescein angiography (FA) and optical coherence tomography were performed to confirm the diagnosis and identify the associated retinal disorder (Fig. 2A, B). Following the ocular examination, all patients were referred to a neurologist or cardiologist for systemic evaluation of atherosclerotic risk factors including cerebrovascular and cardiovascular disease, diabetes mellitus, hyperlipidemia, hypertension, and carotid artery stenosis. Carotid artery patency (including carotid vessels) was determined through carotid Doppler ultrasound sonography or brain magnetic resonance imaging and magnetic resonance angiography.
To evaluate changes in retinal blood flow, we repeated FA in intervals of 1 to 3 months until complete retinal arterial reperfusion was achieved. Reperfusion was reassessed on follow-up FA images in the event of significantly reduced arm-to-retina time and arteriovenous passage time.
In total, 12 patients (10.9%, 10 men and 2 women) out of 110 CRAO patients developed NVI (Fig. 3). Demographic and systemic characteristics of the patients are presented in Table 1. Ophthalmologic features of eyes that developed NVI or NVG after CRAO attack are reported in Table 2. The median age at CRAO diagnosis was 66 years (range, 43 to 88 years). Three patients (3 of 12, 27.3%) showed severe narrowing in the ipsilateral carotid artery and were diagnosed as having both CRAO and OIS. The other patients had no additional ischemic retinal conditions associated with NVI, including PDR, CRVO, or carotid artery stenosis. Patients with diabetes (case numbers 1-5 and 12) were diagnosed with mild or moderate non-PDR.
The mean time from onset of CRAO incident to diagnosis of NVI was 3.0 months (range, 1 week to 15 months) (Fig. 4). NVI was detected in nine patients on routine follow-up examination. However, three patients (case numbers 4-6) demonstrated NVI at the initial visit following CRAO attack for decreased visual acuity and ipsilateral ocular pain.
Neither panretinal photocoagulation (PRP) nor intravitreal bevacizumab injection (IVB) was performed prior to the development of NVI in any patient. After NVI detection, two patients (2 of 12, 16.7%) underwent only PRP, three patients (3 of 12, 25 %) underwent only IVB, and five patients (5 of 12, 42.7%) underwent combined PRP and IVB as treatment. NVI was successfully resolved in seven of ten treated patients (70.0%). In three cases (case numbers 3-5), NVI failed to resolve, IOP was not controlled with medication, and Ahmed valve implantation surgery was needed. No patient with NVI progressed to NVG. Seven patients (7 of 110, 6.4%) were diagnosed with NVG. All follow-up FA evaluations (10 of 12) demonstrated that reperfusion was not achieved when NVI was initially observed (Fig. 2A, B). Choroidal perfusion was also evaluated with FA, and ophthalmic arterial stenosis was confirmed during intra-arterial thrombolysis. As a result, three patients (3 of 12, 25%) showed delayed choroidal perfusion during the initial FA.
Comparisons of clinical parameters between patients with and without NVI are shown in Table 3. Among the group without NVI, two patients did not receive carotid arterial patency evaluations, and three patients refused to undergo follow-up FA. There were no significant differences in mean age, hypertension, severe carotid artery stenosis, coronary arterial disease, previous stroke history, smoking, or dyslipidemia between the two groups. However, diabetes mellitus was more common in patients with NVI than without NVI (50.0% vs. 17.3%, p = 0.017). All patients with NVI who underwent follow-up FA failed to achieve retinal artery reperfusion. Only five of 95 patients without NVI failed to achieve reperfusion on the last follow-up FA images (0% vs. 94.7%, p < 0.001). The median time of their last follow-up FA was 3 months from the onset of CRAO (range, 1 month to 5 years). Kaplan-Meier survival analysis showed that the cumulative incidence rates were 5.5% at 3 months, 7.3% at 6 months, and 10.9% at 15 months (Fig. 3).
Our study demonstrated an incidence of NVI following acute CRAO of 10.9%. Previous studies have reported a prevalence of ocular NV post CRAO ranging from 3.0% to 28.2% [1234]. Our result is within the range of previous reports.
However, in this study, the mean time to NV was 3 months following acute CRAO, which is longer than the 5 and a half weeks found in a different prospective study [5]. The difference may be due to discrepancies in the degree of ischemia and in the timing of follow-up visits between the two studies.
NV usually occurs in chronic retinal ischemic diseases, such as PDR, CRVO, and OIS [7]. There is much debate regarding the prevalence and pathogenesis of NVI following CRAO. Hayreh and Podhajsky [3] have attributed NVI and NVG associated with CRAO to underlying atherosclerotic carotid artery disease. Hayreh et al. [8] did not find a cause-effect relationship between CRAO and ocular NV in their cohort of 232 patients with CRAO. Conversely, Rudkin et al. [4] demonstrated a clear empirical correlation between thromboembolic CRAO itself and NV, and the majority of patients in that study who developed NV did not have diabetes or an association with hemodynamically significant stenosis of the carotid artery that could account for NV. Our results also revealed that CRAO may induce NVI even without atherosclerotic carotid artery disease or diabetes. The most important factor for the development of NVI was a failure to gain reperfusion and the resultant chronic retinal ischemia. We theorize that severe carotid arterial stenosis may be one of the causes of failure to gain reperfusion. However, other causes including ophthalmic artery stenosis and central retinal artery obstruction may lead to chronic retinal ischemia and NVI. Following a CRAO attack, a patient's eyes usually achieve retinal arterial reperfusion within 1 month and follow a stable disease course without ocular complications. However, in certain cases, CRAO may induce NVI and sometimes induce NVG caused by chronic ischemia due to reperfusion failure. From our study results, we propose that some eyes with acute CRAO may develop chronic CRAO. OIS refers to ischemic retinopathy caused by severe carotid artery diseases [9]. OIS may be one of the causes of chronic CRAO. Therefore, NVI or NVG following acute CRAO without restored perfusion may initially be due to chronic CRAO rather than OIS.
NVI and NVG are highly correlated with retinal ischemia, and stimulate the secretion of vascular endothelial growth factor [10]. Vascular endothelial growth factor levels are reduced after PRP in patients with ischemic retinal disorders [11]. Currently, PRP is the gold standard for initial treatment. Duker and Brown [11] reported regression in 65% of patients after PRP for NVI following CRAO. Sagong et al. [2] reported three cases that achieved successful treatment of NVG secondary to CRAO with combined PRP and IVB. Two of three patients without NVI resolution underwent only IVB and the other patient refused additional IVB. In our case series, there was no progression from NVI to NVG after PRP or IVB. Therefore, PRP combined with IVB could be a valuable management tool for treatment of NVI associated with CRAO.
There is no consensus regarding the proper follow-up regimen after CRAO because of the uncertain course of ocular complications. In CRVO patients, NVG is a well-recognized complication, sometimes called 100-day glaucoma. Current guidelines recommend close follow-up for patients with CRVO to measure IOP and detect NV, especially in the first 6 months [12]. Rudkin et al. [4] suggested review of all patients with CRAO at regular intervals, as early as 2 weeks and up to 4 months post CRAO. However, the study had a small number of patients (33), retinal artery reperfusion was not assessed, and a comparison of clinical parameters with patients without NV was not made.
Our study shows that NVI after CRAO tends to occur around 3 months (range, 1 week to 15 months). We suggest that careful examination with follow-up FA is necessary to confirm reperfusion up to 3 months after the initial event, particularly when the patient has diabetes. If failure to gain reperfusion is observed, monthly check-ups to detect NVI and IOP elevation are required and prophylactic PRP should be considered.
The limitations of our study include its retrospective nature, which has inherent selection bias and a small number of patients who developed NVI. In addition, the follow-up schedule after CRVO incidence was irregular among patients. Additionally, another limitation of our study is that we did not perform indocyanine green angiography to rule out ophthalmic artery occlusion. Therefore, rare cases of ophthalmic artery stenosis or occlusion might have been included in the CRAO cases. Considering that a clear distinction between CRAO and ophthalmic artery occlusion is difficult and sometimes impossible, we believe that the lack of indocyanine green angiography should not alter the primary conclusion of our study. The strength of our study is that we included a large number of CRAO patients, and therefore a reliable incidence of NVI due to CRAO was calculated from our data.
In conclusion, neovascularization of iris and neovascular glaucoma can develop following CRAO attack even without severe carotid artery obstruction. The most important risk factor of anterior segment neovascularization is the failure of retinal arterial reperfusion and the resultant chronic ischemia.
Figures and Tables
 | Fig. 1Flow chart of stuy design and exclusion criteria. CRAO = central retinal artery occlusion; NVI = neovascularization of the iris. |
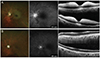 | Fig. 2Case 11. Fundus photography (left), fundus fluorescein angiography (middle), and optical coherence tomography (right) were obtained when the patient initially visited (A) and after 4 months when neovascularization of the iris was detected (B). Text (lower right corner) in the fundus fluorescein angiography indicates the time (54 seconds and 98 seconds) when images were taken after fluorescein infusion. (A) Examination results showed typical acute central retinal artery occlusion, including a cherry red spot. (B) After 4 months, angiography showed delayed retinal arterial perfusion, with inner retinal atrophy from optical coherence tomography. |
 | Fig. 3Kaplan-Meier survival curves following onset of central retinal artery occlusion (CRAO). The cumulative incidences were 5.5% at 3 months, 7.3% at 6 months, and 10.9% at 15 months. NVI = neovascularization of the iris. |
 | Fig. 4Case 11. Neovascularization of the iris was observed 4 months after the initial event of central retinal artery occlusion. |
Table 1
Demographic and systemic characteristics of cases that developed neovascularization of the iris or neovascular glaucoma after central retinal artery occlusion attack

Table 2
Ophthalmologic features of eyes that developed NVI or NVG after CRAO attack

NVI = neovascularization of the iris; NVG = neovascular glaucoma; CRAO = central retinal artery occlusion; VA = visual acuity; IOP = intraocular pressure; HM = hand motions; CF = count fingers; PRP = panretinal photocoagulation; OIS = ocular ischemic syndrome; NLP = no light perception; AVI = Ahmed valve implantation; NA = not assessed; LP = light perception; IA = intra-arterial.
Acknowledgements
This study was supported by a grant (CCP-13-02-KIST) from Convergence Commercialization Projec t of National Research Council of Science and Technology, Seoul, Korea.
References
1. Park SJ, Choi NK, Seo KH, et al. Nationwide incidence of clinically diagnosed central retinal artery occlusion in Korea, 2008 to 2011. Ophthalmology. 2014; 121:1933–1938.
2. Sagong M, Kim J, Chang W. Intravitreal bevacizumab for the treatment of neovascular glaucoma associated with central retinal artery occlusion. Korean J Ophthalmol. 2009; 23:215–218.
3. Hayreh SS, Podhajsky P. Ocular neovascularization with retinal vascular occlusion. II: Occurrence in central and branch retinal ar tery occlusion. Arch Ophthalmol. 1982; 100:1585–1596.
4. Rudkin AK, Lee AW, Chen CS. Ocular neovascularization following central retinal artery occlusion: prevalence and timing of onset. Eur J Ophthalmol. 2010; 20:1042–1046.
5. Duker JS, Sivalingam A, Brown GC, Reber R. A prospective study of acute central retinal artery obstruction: the incidence of secondary ocular neovascularization. Arch Ophthalmol. 1991; 109:339–342.
6. Ahn SJ, Kim JM, Hong JH, et al. Efficacy and safety of intra-arterial thrombolysis in central retinal artery occlusion. Invest Ophthalmol Vis Sci. 2013; 54:7746–7755.
7. Sivak-Callcott JA, O'Day DM, Gass JD, Tsai JC. Evidence-based recommendations for the diagnosis and treatment of neovascular glaucoma. Ophthalmology. 2001; 108:1767–1776.
8. Hayreh SS, Podhajsky PA, Zimmerman MB. Retinal artery occlusion: associated systemic and ophthalmic abnormalities. Ophthalmology. 2009; 116:1928–1936.
9. Mendrinos E, Machinis TG, Pournaras CJ. Ocular ischemic syndrome. Surv Ophthalmol. 2010; 55:2–34.
10. Aiello LP, Avery RL, Arrigg PG, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994; 331:1480–1487.
11. Duker JS, Brown GC. The efficacy of panretinal photocoagulation for neovascularization of the iris after central retinal artery obstruction. Ophthalmology. 1989; 96:92–95.
12. Natural history and clinical management of central retinal vein occlusion: the Central Vein Occlusion Study Group. Arch Ophthalmol. 1997; 115:486–491.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download