Abstract
Purpose
We evaluated fundus and fluorescein angiography (FAG) findings and characteristics that can help distinguish nonarteritic anterior ischemic optic neuropathy (NAION) from optic neuritis (ON).
Methods
Twenty-three NAION patients and 17 ON with disc swelling patients were enrolled in this study. We performed fundus photography and FAG. The disc-swelling pattern, hyperemia grade, presence of splinter hemorrhages, cotton-wool spots, artery/vein ratio and degree of focal telangiectasia were investigated. The FAG findings for each patient were compared with respect to the following features: the pattern of disc leakage in the early phase, arteriovenous (artery/vein) transit time (second), and the presence and pattern of the filling delay.
Results
Cotton-wool spots, focal telangiectasia, and venous congestion were more common in the affected eyes of NAION patients. Upon FAG, 76.5% of the patients in the ON group exhibited normal choroidal circulation. However, 56.5% of patients in the NAION group demonstrated abnormal filling defects, such as peripapillary, generalized, or watershed zone filling delays.
Unilateral optic nerve dysfunction with optic disc swelling has been reported in various disorders, such as nonarteritic anterior ischemic optic neuropathy (NAION), optic neuritis (ON), compressive optic neuropathy, infiltrative optic neuropathy, and retinal vein occlusion. Among these, NAION and ON are common causes of unilateral disc swelling disorders [1]. NAION and ON, especially papillitis, which is an intraocular ON, frequently have overlapping clinical profiles, and it can be difficult to differentiate them on clinical grounds at the time of the initial presentation [2].
To differentiate between NAION and ON, the patients' age, sex, associated symptoms (such as pain on ocular movement) and systemic diseases (such as hypertension, diabetes mellitus, multiple sclerosis, and neuromyelitis optica) should be carefully investigated. Ancillary examinations, including visual field tests, color vision tests, and imaging tests, are also very helpful in differentiating between the two diseases. Although altitudinal optic disc swelling, pallor, arterial attenuation, and splinter hemorrhage are more suggestive of a diagnosis of NAION [3], it is difficult to diagnose NAION during the fundus examination in cases that do not show definite splinter hemorrhage and altitudinal swelling.
Fluorescein angiography (FAG) is a useful method for evaluating the optic disc. In cases of NAION, FAG typically reveals delayed optic disc filling and circular or localized peripapillary choroid filling delays [45]. However, few published reports have examined FAG findings in ON, and there have been no comparative studies directly conducted between NAION and ON. Therefore, we evaluated the fundus findings and FAG characteristics that can help distinguish NAION from ON.
We reviewed the medical records of patients who were diagnosed with NAION and ON with disc swelling from May 2008 to April 2014. Acute unilateral ON with disc swelling included acute painful visual loss for 8 days or less, a relative afferent pupillary defect, a visual field defect and a color vision defect in the affected eye. Seventeen ON patients (8 men and 9 women) were enrolled. Magnetic resonance imaging (MRI) was performed in 15 out of the 17 patients and produced an enhancement of the involved optic nerve in 14 of these patients. The two patients who did not undergo MRI and the patient who had normal MRI findings had a final visual acuity greater than 0.8. The inclusion criteria for NAION were symptoms lasting less than 14 days at the baseline eligibility visit, a painless visual loss from 20 / 64 to light perception in the affected eye, visual field defects consistent with optic neuropathy and less than or equal to -3.0 dB (16 patients had an altitudinal defect, five patients had a diffuse decreased sensitivity, and two patients had quadrianopsia-like lesion), an relative afferent pupillary defect and disc edema. We selected only unilateral NAION patients in order to allow for comparison with the healthy eye. A total of 23 NAION patients (12 men and 11 women) were enrolled. This research study was reviewed and approved by the institutional review board of the Kim's Eye Hospital, Seoul, Korea.
We investigated the presence of diabetes mellitus and hypertension and performed full ophthalmologic examinations, including assessments of the best-corrected visual acuity and intraocular pressure, slit-lamp examination, and pupillary reaction and fundus examinations. The refractive errors were measured by manifest refraction. We used the spherical equivalent to analyze the differences between groups.
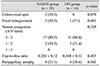
Fundus photographs and FAG images were obtained using a digital fundus camera (CF-60DSi and CF-60UVi; Canon, Tokyo, Japan). In the fundus photographs, we analyzed features of the optic disc, including the area showing disc swelling (no swelling, focal superior, focal inferior, or diffuse) and the hyperemia grade. The degree of disc hyperemia with reference to the contralateral normal disc was graded into four categories: 0, no hyperemia (similar to the contralateral normal disc or to other age-matched normal discs); +1, minimal hyperemia (pinkish disc); +2, moderate hyperemia (pinkish to reddish disc); and +3, marked hyperemia (reddish disc). The presence of splinter hemorrhages, cotton-wool spots, and focal telangiectasia was also investigated. Venous congestion was measured using the artery/vein (A/V) ratio. On the basis of these ratios, venous congestion was divided into three classes: (1) 2 : 3, (2) 1 : 2, and (3) 1 : 3. The cup-to-disc ratio and peripapillary atrophy in the non-affected eye were investigated as well.
The FAG findings for each patient were compared with respect to the following features: the pattern of disc leakage in the early phase (diffuse or altitudinal), A/V transit time (second), and the presence and pattern of the filling delay. The FAG patterns were categorized as follows: generalized filling delay, filling delay in the watershed zone, and a localized filling delay in the peripapillary choroid.
Statistical analyses were performed using SPSS ver. 14.0 (SPSS Inc., Chicago, IL, USA). The Student t-test was used to analyze the statistical differences in the cup-to-disc ratio, as determined on the basis of the fundus photographs and A/V transit time during FAG. Other parameters were analyzed using Fisher's exact test and analysis of variance.
The mean age of the study participants was 56.9 ± 9.4 years in the NAION group and 47.8 ± 15.2 years in the ON group (p = 0.038). The rates of diabetes mellitus, hypertension, and refractive error were similar between the two groups (Table 1). Fundus photography revealed a significant difference between the affected eye and the non-affected eye in the NAION group. In other words, cotton-wool spots, focal telangiectasia, and venous congestion were more common in the affected eyes of NAION patients (p = 0.048, p = 0.015, and p < 0.001, respectively). No such difference was observed in the ON group (Table 2).
Although the incidence of disc hyperemia and disc-swelling patterns were similar in the NAION and ON groups, other parameters, including splinter hemorrhage, cotton-wool spots, and focal telangiectasia, were more common in the NAION group. Severe venous congestion (>1 : 2) was also observed more frequently in the NAION group (Table 3). The frequencies of abnormal fundus findings, such as cotton-wool spots, focal telangiectasia, and venous congestion in the non-affected eyes, were also similar in both groups (Table 4). In addition, these two groups had similar cup-to-disc ratios and rates of peripapillary atrophy.
A/V transit time was similar in both groups (NAION, 12.50 ± 3.46 seconds; ON, 10.55 ± 2.83 seconds, p = 0.371). However, diffuse disc leakage in the early phase was more common in the NAION group (69.6% vs. 52.9%). The rates of the filling delay were slightly different in both groups. Notably, 76.5% of the patients in the ON group exhibited normal choroidal circulation. However, 56.5% of patients in the NAION group displayed abnormal filling defects, such as peripapillary, or generalized or watershed zone filling delays (chi-square test, p = 0.010) (Fig. 1).
FAG findings and the results of fundus photography could provide valuable information about differentiating a diagnosis between NAION and ON. In ON, the present result had a 1 : 1 ratio of females to males. In contrast, a previous ON treatment trial documented a female predominance (2.7 : 1) [6]. However, the ratio of male patients increased in Asian studies [78]. As previous reports have shown, the average age in the NAION group was greater than that in the ON group. Diabetes mellitus and hypertension were more common in NAION patients, but this trend was not significant. Diabetes mellitus and hypertension were identified as risk factors for NAION (p = 0.074 and 0.080, respectively) [9]. These differences might have been statistically significant if more participants had been included in the study. The incidence of refractive errors, which could affect parameters such as the cup-to-disc ratio and peripapillary atrophy, were similar in both groups.
Cotton-wool spots are caused by acute ischemic retinal nerve fiber injury and focal telangiectasia, which is defined as a dilation of the small blood vessels due to vascular insufficiency. Venous congestion is another sign of vascular insufficiency. These three factors were commonly observed in the affected eye in patients with NAION, and these results suggest that vascular insufficiency was associated to NAION. Moreover, the affected and non-affected eyes were similar in the ON group. Thus, cotton-wool spots, focal telangiectasia, and venous congestion represent clues that could be used to differentiate NAION from ON.
In this study, the degrees of disc hyperemia and disc swelling were similar in the affected eyes of both groups. The superior optic nerve head was more vulnerable in NAION; at the same time, focal superior swelling was observed in more than half of the patients [10]. However, the present results contained only two focal instances of superior optic disc swelling. The reason for this difference may be explained by the fact that all of the patients who enrolled were in the acute stage (NAION group, within 14 days), which may have affected the pattern of disc swelling and could explain this discrepancy between studies. The high number of acute stage patients may also explain why the disc findings (other than the rate of splinter hemorrhages) did not differ between the groups. Optic disc swelling decreased over time in both the NAION and ON groups. A different optic disc-swelling may be detected in the subacute state.
Cotton wool spots, focal telangiectasia, and venous congestion were more common in patients with NAION. These results suggest that circular vascular insufficiency was more severe in NAION. Although NAION is caused by infarction of the laminar or retrolaminar portion of the optic nerve head, which is supplied by the short posterior ciliary arteries, the presumed pathophysiology of ON is inflammation and demyelination of the optic nerve [1112]. Diabetes mellitus and hypertension may also induce these factors [13]; however, the non-affected eyes were similar in the NAION and ON groups. Rath et al. [14] reported statistically significant differences in the morphology of optic disc atrophy after NAION compared to that of ON patients with respect to three parameters: the area of involvement, the degree of pallor, and the A/V ratio. These features (including cotton-wool spots, focal telangiectasia, and venous congestion) may be clues that can be used to diagnose NAION.
Studies using FAG have reported that patients with NAION had optic disc leakage but only focal delays, with times similar to those of the normal controls [15]. The present study also showed no difference in the A/V time between the groups. Various types of choroidal insufficiencies, such as localized filling delays, generalized filling delays and watershed zone pattern delays, were observed in the patients with NAION [5]. The current study presented a significantly different pattern of filling delay. During the early stages of anterior ischemic optic neuropathy, there was a significantly delayed and poor filling of the choroid by the posterior ciliary arteries [16]. In addition, vascular occlusion in NAION is not limited within the choroidal circulation because the choroid majorly supplies the anterior prelaminar and laminar layers [17]. In other words, choroidal insufficiencies, including localized filling delays, generalized filling delays and watershed zone pattern delays, could be helpful in differentiating NAION from ON.
Figures and Tables
 | Fig. 1Fluorescein angiography findings between nonarteritic anterior ischemic optic neuropathy (NAION) patients and optic neuritis (ON) patients. |
References
1. Jung JJ, Baek SH, Kim US. Analysis of the causes of optic disc swelling. Korean J Ophthalmol. 2011; 25:33–36.
2. Rizzo JF 3rd, Lessell S. Optic neuritis and ischemic optic neuropathy: overlapping clinical profiles. Arch Ophthalmol. 1991; 109:1668–1672.
3. Warner JE, Lessell S, Rizzo JF 3rd, Newman NJ. Does optic disc appearance distinguish ischemic optic neuropathy from optic neuritis? Arch Ophthalmol. 1997; 115:1408–1410.
4. Arnold AC, Badr MA, Hepler RS. Fluorescein angiography in nonischemic optic disc edema. Arch Ophthalmol. 1996; 114:293–298.
5. Shin SY, Kim DS, Ko MK. Fluorescein angiographic features of choroidal insufficiency in anterior ischemic optic neuropathy. Korean J Ophthalmol. 1999; 13:100–104.
6. Beck RW, Cleary PA, Anderson MM Jr, et al. A randomized, controlled trial of corticosteroids in the treatment of acute optic neuritis: the Optic Neuritis Study Group. N Engl J Med. 1992; 326:581–588.
7. Du Y, Yang J, Li JJ, et al. Unilateral optic neuritis in a Chinese population in three centers. J Clin Neurosci. 2011; 18:902–904.
8. Wakakura M, Minei-Higa R, Oono S, et al. Baseline features of idiopathic optic neuritis as determined by a multicenter treatment trial in Japan: Optic Neuritis Treatment Trial Multicenter Cooperative Research Group (ONMRG). Jpn J Ophthalmol. 1999; 43:127–132.
9. Salomon O, Huna-Baron R, Kurtz S, et al. Analysis of prothrombotic and vascular risk factors in patients with nonarteritic anterior ischemic optic neuropathy. Ophthalmology. 1999; 106:739–742.
10. Contreras I, Noval S, Rebolleda G, Munoz-Negrete FJ. Follow-up of nonarteritic anterior ischemic optic neuropathy with optical coherence tomography. Ophthalmology. 2007; 114:2338–2344.
11. Kerr NM, Chew SS, Danesh-Meyer HV. Non-arteritic anterior ischaemic optic neuropathy: a review and update. J Clin Neurosci. 2009; 16:994–1000.
12. Pau D, Al Zubidi N, Yalamanchili S, et al. Optic neuritis. Eye (Lond). 2011; 25:833–842.
13. Brown GC, Brown MM, Hiller T, et al. Cotton-wool spots. Retina. 1985; 5:206–214.
14. Rath EZ, Rehany U, Linn S, Rumelt S. Correlation between optic disc atrophy and aetiology: anterior ischaemic optic neuropathy vs optic neuritis. Eye (Lond). 2003; 17:1019–1024.
15. Oto S, Yilmaz G, Cakmakci S, Aydin P. Indocyanine green and fluorescein angiography in nonarteritic anterior ischemic optic neuropathy. Retina. 2002; 22:187–191.
16. Hayreh SS, editor. Anterior ischemic optic neuropathy. Berlin: Springer-Verlag Berlin Heidelberg;2012. p. 72–73.
17. Miller NR, Newman NJ, Biousse V, Kerrison JB, editors. Walsh and Hoyt's clinical neuro-ophthalmology. 6th ed. Philadelphia: Lippincott Williams & Wilkins;2005. p. 349–384.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download