Abstract
Purpose
Intravitreal anti-vascular endothelial growth factor (anti-VEGF) is the first choice of treatment for age-related macular degeneration. However, quite a few eyes treated using conventional dose anti-VEGF (CDAV) have persistent pigment epithelial detachment (PED) on optical coherence tomography. This study investigated the efficacy and safety of high dose anti-VEGF (HDAV) for refractory PED.
Methods
In this retrospective study, 31 eyes of neovascular age-related macular degeneration patients with persistent PED findings despite six or more intravitreal injections of CDAV (bevacizumab 1.25 mg or ranibizumab 2.5 mg) were analyzed. Changes in visual outcome, central foveal thickness, and PED height were compared before and after HDAV (bevacizumab 5.0 mg) for these refractory PED cases.
Results
The mean age of patients was 67.7 years. The number of CDAV injections was 12.1. The number of HDAV injections was 3.39. Best-corrected visual acuity in logarithm of the minimum angle of resolution before and after HDAV was 0.49 and 0.41 (p < 0.001), respectively. Central foveal thickness before and after HDAV was 330.06 and 311.10 µm (p = 0.125), respectively. PED height before and after HDAV was 230.28 and 204.07 µm (p = 0.014), respectively. There were no serious adverse reactions in all the eyes.
Age-related macular degeneration (AMD) is the most common cause of irreversible visual impairments [12]. Choroidal neovascularization (CNV) and subsequent vascular leakage is the main cause of severe visual impairment in AMD [3] and increased levels of vascular endothelial growth factor (VEGF) is the most important factor contributing to the development of CNV [45]. To date, many studies including the ANCHOR and MARINA trials have shown good efficacy with conventional dose anti-VEGF (CDAV) treatment of CNV in AMD [67891011].
Pigment epithelial detachment (PED) is a pathological process in which the retinal pigment epithelium (RPE) separates from the underlying Bruch's membrane [1213]. PED is a frequent finding in patients with AMD. The association between PED and neovascular AMD is notable as it is a marker of disease severity, progression, and in some cases, resistance to treatment. Despite several therapeutic efforts, PED represents a significant cause of visual morbidity in patients with neovascular AMD and remains a significant treatment challenge. The therapeutic effect for PED is not consistent among previous studies and a decrease in visual acuity of more than 15 letters is still seen in 5% to 15% of treated patients [71114]. The reason for the inconsistent therapeutic effect may be due to the less than optimal dosage of CDAV in PED treatment. Increasing the dosage of anti-VEGF may be required for PED non-responsive to CDAV. An animal study performed on monkeys supports the evidence for high dose anti-VEGF (HDAV) intended to reach high concentrations, in which the intravitreal maximal concentration of ranibizumab was 3.6 fold higher in the 2.0 mg dosage group compared to the 0.5 mg dosage group [15].
This study sought to determine the effects of high dose bevacizumab for refractory PED previously, but unsuccessfully treated with CDAV. Specifically, the changes in visual acuity, central foveal thickness, and PED height after CDAV and HDAV were investigated.
The charts of patients diagnosed with neovascular AMD patients from the Retina Center of Nune Eye Hospital were reviewed. This study was approved by the institutional review board of the Nune Eye Hospital.
The following were the inclusion criteria used to identify subjects: patients diagnosed with neovascular AMD at an age of more than 50 years; and patients with persistent PED on spectral domain-optical coherence tomography (SD-OCT; Spectralis OCT, Heidelberg Engineering, Heidelberg, Germany) despite having six or more prior injections at 4- to 6-week intervals with CDAV (bevacizumab [Avastin, 1.25 mg/0.05 mL; Genentech, South San Francisco, CA, USA] or ranibizumab [Lucentis, 0.5 mg/0.05 mL; Genentech]). Patients with diabetic retinopathy, retinal vein occlusion, and other disorders invading the macular region were excluded. Those who had a history of ophthalmological surgery within the previous 3 months were also excluded.
The data from visual acuity tests using an Early Treatment Diabetic Retinopathy Study chart, intraocular pressure measurements, slit lamp examinations, fundus examinations using indirect ophthalmoscope, fundus photography, fluorescein angiography, and SD-OCT were reviewed on all included subjects. Central foveal thickness was measured through a program in SD-OCT. The PED height was measured with a computerized ruler from the base to the top of the PED in the sub fovea.
In the vitreous cavity of each eye, a dosage of increased bevacizumab 5.0 mg/2.0 mL was injected. Proparacain was dropped in the eye and wiped off with 5% betadine. Opening the eyelids with a speculum, a few drops of 5% betadine were added. After a 90-second wait to allow for the 5% betadine to dry, the pars plana was punctured 3 to 4 mm away from the limbus using an insulin syringe and a 30-guage needle and the prepared bevacizumab was slowly injected. The injected site was pressed with a cotton swab to prevent withdrawal of the medication. All subjects were given a drop of moxifloxacin 0.5% eyedrop (Vigamox; Alcon Laboratories, Fort Worth, TX, USA).
On the first day, seventh day, and fourth week of injections, subjects visited the clinic and underwent slit lamp examinations, visual acuity tests, and intraocular pressure measurements. Visual acuity testing and SD-OCT at 1-month intervals and angiography at 3-month intervals were performed. The changes in visual acuity, central foveal thickness, and PED height before and after CDAV and HDAV were analyzed using the paired t-test (SPSS ver. 15.0; SPSS Inc., Chicago, IL, USA).
A total of 22 males and 9 females were included and 31 eyes analyzed. The mean age was 67.7 ± 6.4 years (range, 56 to 82 years). Before HDAV, the mean number and duration of CDAV was 12.1 ± 8.6 times (range, 7 to 34 times) and 26.1 ± 21.2 months (range, 9 to 60 months). The mean number and duration of HDAV (bevacizumab 5.0 mg) was 3.4 ± 0.8 times (range, 3 to 7 times) and 4.1 ± 1.0 months (range, 3 to 7 months) (Table 1).
After CDAV, the best-corrected visual acuity in logarithm of the minimum angle of resolution changed from 0.45 ± 0.48 to 0.49 ± 0.43 (mean best-corrected visual acuity change, 0.05 ± 0.24; p = 0.278) with no significant difference. After HDAV, the best-corrected visual acuity was 0.41 ± 0.42 showing a significant improvement with a mean of 0.08 ± 0.12 (p < 0.001) (Fig. 1).
After CDAV, the mean central foveal thickness increased from 321.03 ± 90.01 to 330.06 ± 106.01 µm with no significant difference (mean thickness change, 9.03 ± 77.48 µm; p = 0.521). After HDAV, the mean central foveal thickness decreased from 330.06 ± 106.01 to 311.10 ± 112.73 µm with no significant difference (mean thickness change, 18.79 ± 66.83 µm; p = 0.125) (Fig. 2).
After CDAV, the mean PED height decreased from 277.46 ± 199.44 to 230.28 ± 134.36 µm with no significant difference (mean height change, 47.17 ± 39.44 µm; p = 0.529). After HDAV, the mean PED height significantly decreased from 230.28 ± 134.36 to 204.07 ± 142.28 µm (mean height change, 18.79 ± 66.83 µm; p = 0.014) (Fig. 3). Quantitative OCT analysis showed a decrease in the PED height of more than 50 µm in 51.6% (16 / 31) of eyes. Complete resolution of PED was seen in two eyes.
During the follow-up period, side effects such as a conjunctival hemorrhage were seen in 9.4% of patients, vitreous floaters in 6.5%, increased intraocular pressure in 3.2%, and a foreign body sensation in 12.9%. However, systemic problems were not noted (Table 2).
A 64-year-old man with AMD complicated by PED in the right eye and a visual acuity of 20 / 100 was injected with CDAV 28 times (ranibizumab 0.5 mg) during a 40-month period. Subfoveal-vascularized PED persisted with no improvement in visual acuity (Fig. 4A). Afterwards, bevacizumab 5.0 mg was injected monthly. Three months later, the PED height on SD-OCT was found to be decreased and visual acuity improved to 20 / 80 (Fig. 4B).
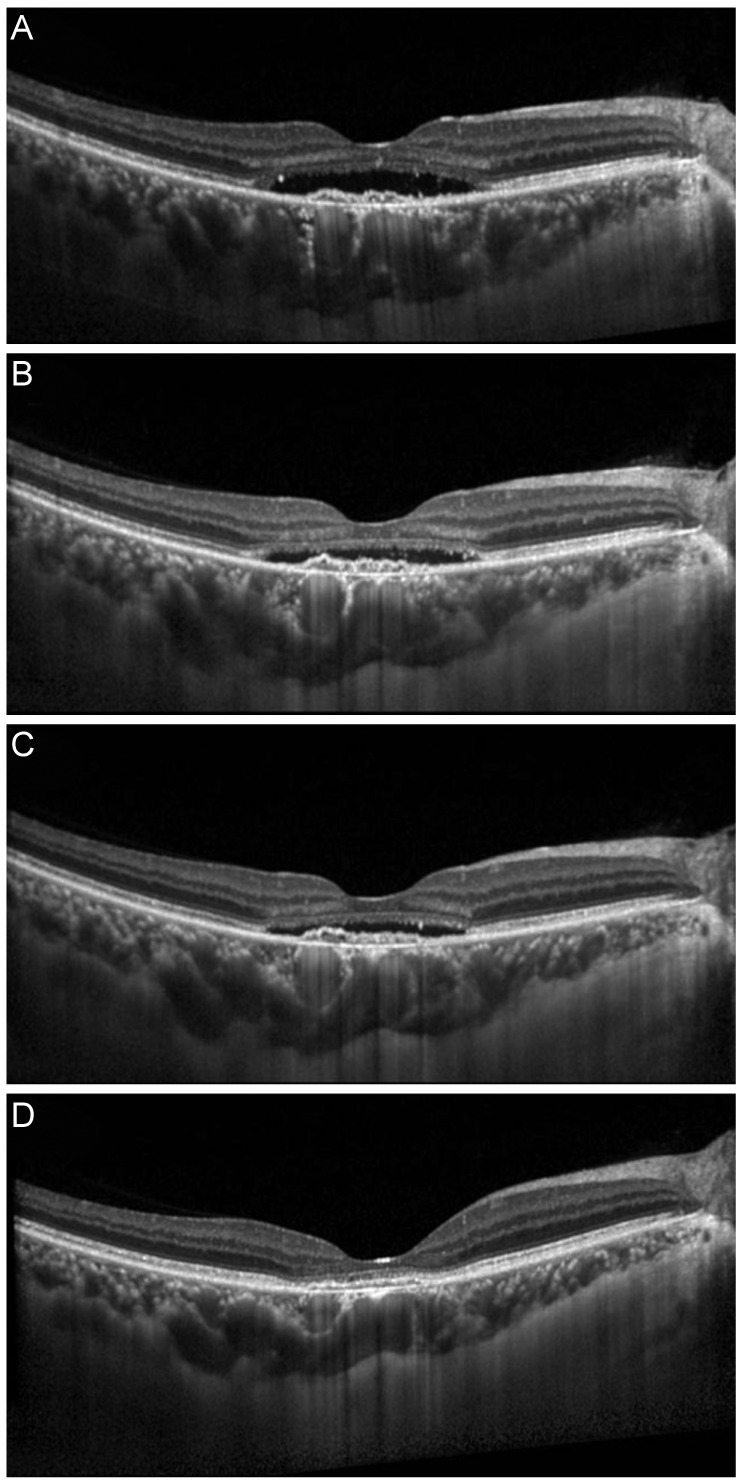
A 72-year-old woman with AMD complicated by PED in the left eye was injected with CDAV 10 times (ranibizumab 0.5 mg × 3, bevacizumab 1.25 mg × 7) during a 10-month period. After several injections of CDAV, visual acuity slightly improved from 20 / 125 to 20 / 100, but PED findings on SD-OCT persisted (Fig. 5A-5C). After two monthly injections of bevacizumab 5.0 mg, PED was found to be significantly decreased and visual acuity improved to 20 / 50 (Fig. 5D).
The pathogenesis of PED formation in AMD is not completely understood, but likely involves the penetration of CNV through Bruch's membrane into the sub-RPE space with secondary extravasation of fluid or blood, and an increase in hydrostatic pressure that separates the RPE from the underlying Bruch's membrane [12].
The clinical implication of PED is seen in the worsening of visual acuity during its natural course. Nearly 50% of patients with newly diagnosed untreated PED experienced a loss of more than 15 letters during a mean observation period of 1 year [16]. Casswell et al. [17] reported functional worsening in most of their patients with PED after 1 year of observation. However, there is no consensus on the therapeutic effect or even on the need for active treatment for PED. There have been few studies reporting on the effect of anti-VEGF drugs for PED, and of those that have been done, results show varying levels of effectiveness [1819202122].
The optimal dosage of anti-VEGF with the lowest toxicity and highest therapeutic effect for PED treatment is not established. In clinical practice, patients with PED and no improvement on CDAV (bevacizumab 1.25 mg or ranibizumab 0.5 mg) are commonly encountered. According to the report of the CATT Research Group [9], among patients who received ranibizumab 0.5 mg monthly, 50% showed persistent fluid and 7% had residual PED. It is unknown why some eyes with neovascular AMD dry up anatomically with fewer injections of anti-VEGF, but up to half have OCT finding of disease activity even with continuous monthly injection [9].
It is possible that some patients may require a higher concentration of VEGF blockade to achieve disease quies-cence or may have faster clearance of anti-VEGF drug from their vitreous cavity. A higher dosage of anti-VEGF drug should theoretically address both hypothetical mechanisms.
The effect of a higher dosage of anti-VEGF drug for AMD is inconsistent across many studies. Modarres et al. [23] reported that bevacizumab 2.5 mg has the same therapeutic effect as bevacizumab 1.25 mg, but with more toxicity such as vitreous reactions. Wu et al. [24] studied 25 eyes and concluded that bevacizumab 1.25 and 2.5 mg showed similar effects. In the HARBOR study, ranibizumab 2.0 mg did not show improvements in visual acuity compared with conventional dosages [25].
On the other hand, Costa et al. [26] reported improvements in CNV secondary to AMD with bevacizumab 1.5 mg and 2.0 mg compared with 1.0 mg. Chan et al. [27] saw dramatic improvements in vascularized PED patients with ranibizumab 2.0 mg. Recently, the super dose anti-VEGF trials [28] for recalcitrant neovascular AMD patients showed that monthly injected ranibizumab 0.5 mg was associated with anatomical improvements and visual recovery after ranibizumab 2.0 mg. In the PEARL2 trial (unpublished data; Kokame G. Macula Society annual meeting, 2012 Jun, Israel), good results were observed with monthly ranibizumab 2.0 mg for 6 months.
In this study, a decrease in the PED height of more than 50 µm after HDAV treatment was seen in 16 / 31 (51.6%) patients, compared to CDAV treatment (mean 12.1 injections in a mean duration of 26.1 months). However, a substantial proportion of eyes represented by the remainder of the study population (15 / 31, 49.4%) showed an insufficient response. This suggests that PED may represent a common pathway in AMD, rather than a response to reflect the disease's activity, and HDAV may still be an important treatment option in the future for a subset of patients who are more likely to benefit.
There are some reports on the safety of HDAV. Rosenfeld et al. [29] reported that increasing doses up to 2.0 mg ranibizumab were well tolerated and demonstrated a beneficial clinical effect. Manzano et al. [30] injected more than 5.0 mg of bevacizumab in rabbit eyes and observed vitreous inflammation but no signs of retinal toxicity, electrophysiologically or histologically. In several animal studies [303132], rabbits injected with bevacizumb with doses ranging from 1.25 to 5.0 mg showed no toxicity on eletroretinogram or histological examination.
There are some limitations of the present study. First, because this was not a prospective controlled trial, the injections intervals were not consistent in the subjects. Second, this study included a sample size of only 31 eyes and the findings may not be applied generally. Third, the follow-up period of the subjects was relatively short.
This study indicates significant efficacy of HDAV for PED treatment without systemic side effects. The study subjects were a homogenous ethnic group of Koreans. To date, no large studies on the efficacy of HDAV for PED treatment have been conducted and the promising results from the present study may encourage prospective studies in a larger group.
In conclusion, the administration of higher anti-VEGF dosages for PED in AMD patients who do not respond to conventional dosages is an option worth considering. Prospective case control studies should be done to validate this treatment.
References
1. Klein R, Peto T, Bird A, Vannewkirk MR. The epidemiology of age-related macular degeneration. Am J Ophthalmol. 2004; 137:486–495. PMID: 15013873.

2. Bressler NM, Bressler SB, Congdon NG, et al. Potential public health impact of Age-Related Eye Disease Study results: AREDS report no. 11. Arch Ophthalmol. 2003; 121:1621–1624. PMID: 14609922.
3. Ferris FL 3rd, Fine SL, Hyman L. Age-related macular degeneration and blindness due to neovascular maculopathy. Arch Ophthalmol. 1984; 102:1640–1642. PMID: 6208888.

4. Amin R, Puklin JE, Frank RN. Growth factor localization in choroidal neovascular membranes of age-related macular degeneration. Invest Ophthalmol Vis Sci. 1994; 35:3178–3188. PMID: 7519180.
5. Kliffen M, Sharma HS, Mooy CM, et al. Increased expression of angiogenic growth factors in age-related maculopathy. Br J Ophthalmol. 1997; 81:154–162. PMID: 9059252.

6. Avery RL, Pieramici DJ, Rabena MD, et al. Intravitreal bevacizumab (Avastin) for neovascular age-related macular degeneration. Ophthalmology. 2006; 113:363–372.e5. PMID: 16458968.

7. Fung AE, Lalwani GA, Rosenfeld PJ, et al. An optical coherence tomography-guided, variable dosing regimen with intravitreal ranibizumab (Lucentis) for neovascular age-related macular degeneration. Am J Ophthalmol. 2007; 143:566–583. PMID: 17386270.

8. Chan CK, Meyer CH, Gross JG, et al. Retinal pigment epithelial tears after intravitreal bevacizumab injection for neovascular age-related macular degeneration. Retina. 2007; 27:541–551. PMID: 17558314.

9. CATT Research Group. Martin DF, Maguire MG, et al. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011; 364:1897–1908. PMID: 21526923.

10. Brown DM, Kaiser PK, Michels M, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006; 355:1432–1444. PMID: 17021319.

11. Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006; 355:1419–1431. PMID: 17021318.

12. Gass JD. Serous retinal pigment epithelial detachment with a notch: a sign of occult choroidal neovascularization. Retina. 1984; 4:205–220. PMID: 6085179.
13. Gass JD. Pathogenesis of tears of the retinal pigment epithelium. Br J Ophthalmol. 1984; 68:513–519. PMID: 6204685.

14. Brown DM, Michels M, Kaiser PK, et al. Ranibizumab versus verteporfin photodynamic therapy for neovascular age-related macular degeneration: two-year results of the ANCHOR study. Ophthalmology. 2009; 116:57–65.e5. PMID: 19118696.

15. Gaudreault J, Fei D, Rusit J, et al. Preclinical pharmacokinetics of Ranibizumab (rhuFabV2) after a single intravitreal administration. Invest Ophthalmol Vis Sci. 2005; 46:726–733. PMID: 15671306.

16. Pauleikhoff D, Loffert D, Spital G, et al. Pigment epithelial detachment in the elderly: clinical differentiation, natural course and pathogenetic implications. Graefes Arch Clin Exp Ophthalmol. 2002; 240:533–538. PMID: 12136282.
17. Casswell AG, Kohen D, Bird AC. Retinal pigment epithelial detachments in the elderly: classification and outcome. Br J Ophthalmol. 1985; 69:397–403. PMID: 2408659.

18. Inoue M, Arakawa A, Yamane S, Kadonosono K. Variable response of vascularized pigment epithelial detachments to ranibizumab based on lesion subtypes, including polypoidal choroidal vasculopathy. Retina. 2013; 33:990–997. PMID: 23446653.

19. Parodi MB, Iacono P, Papayannis A, et al. Intravitreal ranibizumab for pigment epithelium detachment with subfoveal occult choroidal neovascularization: a prospective 24-month case series. Am J Ophthalmol. 2013; 155:103–108.e2. PMID: 23022164.

20. Bolz M, Michels S, Geitzenauer W, et al. Effect of systemic bevacizumab therapy on retinal pigment epithelial detachment. Br J Ophthalmol. 2007; 91:785–789. PMID: 17050580.

21. Chen E, Kaiser RS, Vander JF. Intravitreal bevacizumab for refractory pigment epithelial detachment with occult choroidal neovascularization in age-related macular degeneration. Retina. 2007; 27:445–450. PMID: 17420696.

22. Ach T, Hoeh AE, Ruppenstein M, et al. Intravitreal bevacizumab in vascular pigment epithelium detachment as a result of subfoveal occult choroidal neovascularization in age-related macular degeneration. Retina. 2010; 30:1420–1425. PMID: 20543764.

23. Modarres M, Naseripour M, Falavarjani KG, et al. Intravitreal injection of 2.5 mg versus 1.25 mg bevacizumab (Avastin) for treatment of CNV associated with AMD. Retina. 2009; 29:319–324. PMID: 19287288.

24. Wu L, Arevalo JF, Roca JA, et al. Comparison of two doses of intravitreal bevacizumab (Avastin) for treatment of macular edema secondary to branch retinal vein occlusion: results from the Pan-American Collaborative Retina Study Group at 6 months of follow-up. Retina. 2008; 28:212–219. PMID: 18301025.
25. Busbee BG, Ho AC, Brown DM, et al. Twelve-month efficacy and safety of 0.5 mg or 2.0 mg ranibizumab in patients with subfoveal neovascular age-related macular degeneration. Ophthalmology. 2013; 120:1046–1056. PMID: 23352196.

26. Costa RA, Jorge R, Calucci D, et al. Intravitreal bevacizumab for choroidal neovascularization caused by AMD (IBeNA Study): results of a phase 1 dose-escalation study. Invest Ophthalmol Vis Sci. 2006; 47:4569–4578. PMID: 17003454.

27. Chan CK, Abraham P, Sarraf D. High-dose ranibizumab therapy for vascularized pigment epithelial detachment. Eye (Lond). 2012; 26:882–885. PMID: 22576827.

28. Brown DM, Chen E, Mariani A, et al. Super-dose anti-VEGF (SAVE) trial: 2.0 mg intravitreal ranibizumab for recalcitrant neovascular macular degeneration-primary end point. Ophthalmology. 2013; 120:349–354. PMID: 23131717.

29. Rosenfeld PJ, Heier JS, Hantsbarger G, Shams N. Tolerability and efficacy of multiple escalating doses of ranibizumab (Lucentis) for neovascular age-related macular degeneration. Ophthalmology. 2006; 113:623.e1. PMID: 16581423.

30. Manzano RP, Peyman GA, Khan P, Kivilcim M. Testing intravitreal toxicity of bevacizumab (Avastin). Retina. 2006; 26:257–261. PMID: 16508423.

31. Feiner L, Barr EE, Shui YB, et al. Safety of intravitreal injection of bevacizumab in rabbit eyes. Retina. 2006; 26:882–888. PMID: 17031287.

32. Shahar J, Avery RL, Heilweil G, et al. Electrophysiologic and retinal penetration studies following intravitreal injection of bevacizumab (Avastin). Retina. 2006; 26:262–269. PMID: 16508424.

Fig. 1
Change in best-corrected visual acuity (BCVA) in logarithm of the minimum angle of resolution. BCVA was 0.45 ± 0.48 and 0.49 ± 0.43 before and after conventional dose anti-vascular endothelial growth factor (CDAV), respectively. BCVA showed a mean decrease of 0.05 ± 0.24 logarithm of the minimum angle of resolution (p = 0.278) despite CDAV. BCVA was 0.41 ± 0.42 after high dose anti-vascular endothelial growth factor (HDAV). BCVA showed a significant improvement of 0.08 ± 0.12 (*p < 0.001).

Fig. 2
Change in central foveal thickness (CFT). CFT was 321.03 ± 90.01 and 330.06 ± 106.01 µm before and after conventional dose anti-vascular endothelial growth factor (CDAV; mean change of CFT, 9.03 ± 77.48 µm; p = 0.521), respectively. CFT decreased to 311.10 ± 112.73 µm after high dose anti-vascular endothelial growth factor (HDAV; mean change of CFT, 18.79 ± 66.83 µm; p = 0.125).
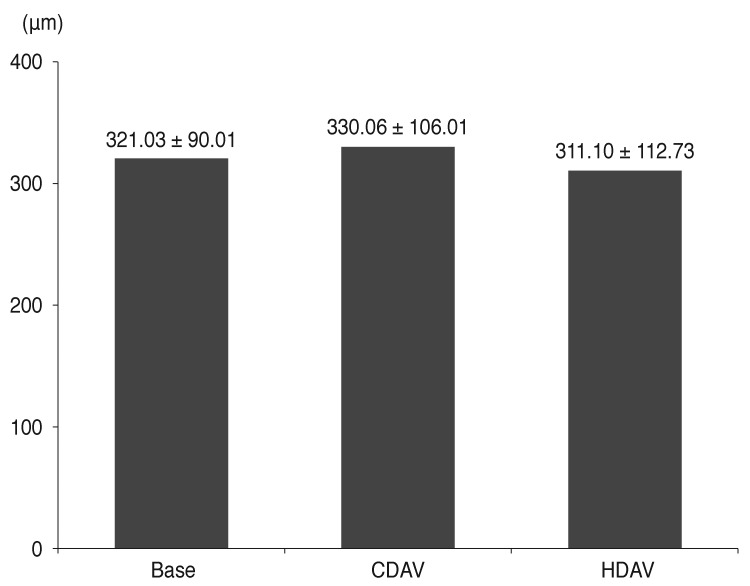
Fig. 3
Change in height of pigment epithelial detachment (PED). The height of the PED was 227.46 ± 199.44 and 230.28 ± 134.36 µm after conventional dose anti-vascular endothelial growth factor (CDAV) treatment, respectively. The PED height decreased by a mean of 47.17 ± 39.44 µm with no statistical significance (p = 0.529). In contrast, eyes in the high dose anti-vascular endothelial growth factor (HDAV) group were found to have a final PED height of 204.07 ± 142.28 µm, which was significantly decreased by a mean of 18.79 ± 66.83 µm (*p = 0.014).

Fig. 4
(A) A 64-year-old man with pigment epithelial detachment (PED) secondary to age-related macular degeneration in right eye with subfoveal choroidal neovascularization was injected with conventional dose anti-vascular endothelial growth factor 28 times (ranibizumab 0.5 mg) during a 40-month period. Subfoveal PED persisted with no improvement of visual acuity. (B) Afterwards, bevacizumab 5.0 mg was injected monthly. Three months later, the marked PED noted on spectral domain-optical coherence tomography had resolved.

Fig. 5
(A) A 72-year-old woman with sub-retinal fluid and pigment epithelial detachment (PED) secondary to age-related macular degeneration in left eye was injected with conventional dose anti-vascular endothelial growth factor nine times (ranibizumab 0.5 mg × 3, bevacizumab 1.25 mg × 7) during a 10-month period. (B) No change in PED after three injections of ranibizumab 0.5 mg. (C) No change in PED after seven injections of bevacizumab 1.25 mg. (D) Significant improvement in PED after 2 monthly injections of bevacizumab 5.0 mg.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download