Abstract
Purpose
This study evaluated the prevalence of ocular toxocariasis (OT) in patients with uveitis of unknown etiology who visited a tertiary hospital in South Korea and assessed the success of serum anti-Toxocara immunoglobulin G (IgG) enzyme-linked immunosorbent assay (ELISA) as a diagnostic test for OT.
Methods
The records of consecutive patients with intraocular inflammation of unknown etiology were reviewed. All participants underwent clinical and laboratory investigations, including ELISA for serum anti-Toxocara IgG. OT was diagnosed based on typical clinical findings. Clinical characteristics, seropositivity, and IgG titers were compared between patients diagnosed with OT and non-OT uveitis. The seropositivity and the diagnostic value of anti-Toxocara IgG was investigated among patients with different types of uveitis.
Results
Of 238 patients with uveitis of unknown etiology, 71 (29.8%) were diagnosed with OT, and 80 (33.6%) had positive ELISA results for serum anti-Toxocara IgG. The sensitivity and specificity of the ELISA test were 91.5% (65 / 71) and 91.0% (152 / 167), respectively. The positive predictive value of the serum anti-Toxocara IgG assay was 81.3%. Among patients with anterior, intermediate, posterior, and panuveitis, the prevalence rates of OT were 8.3%, 47.1%, 44.8%, and 7.1%, respectively; the seropositivity percentages were 18.1%, 47.1%, 43.7%, and 17.9%; and the positive predictive values were 38.5%, 95.8%, 92.1%, and 40.0%. The serum anti-Toxocara IgG titer also significantly decreased following albendazole treatment.
Conclusions
OT is a common cause of intraocular inflammation in the tertiary hospital setting. Considering that OT is more prevalent in intermediate and posterior uveitis, and that the positive predictive value of the anti-Toxocara IgG assay is high, a routine test for anti-Toxocara IgG might be necessary for Korean patients with intermediate and posterior uveitis.
Toxocariasis is one of the most common zoonotic infections caused by larvae of Toxocara canis or Toxocara cati. Human infection can be precipitated by accidental ingestion of eggs due to geophagia or pica or the consumption of raw meat or vegetables contaminated with Toxocara larvae [1]. The development of ocular disease is dependent on the parasite load, the host immune response to the parasites, and migration of the Toxocara larva. Toxocariasis can present clinically as ocular toxocariasis (OT), visceral toxocariasis, or covert toxocariasis [2].
OT occurs when Toxocara larvae migrate through the bloodstream into the posterior compartment of the eye [3]. In general, OT is definitively diagnosed by direct demonstration of worms, larvae, or eggs following biopsy of infected sites. However, it is both difficult and risky to obtain a suitable biopsy specimen from eyes infected with OT. Therefore, the diagnosis of OT is currently based on serologic tests and clinical findings [4]. OT is clinically diagnosed based on the following: (1) typical clinical findings of OT, such as granuloma formation; (2) positive serologic results; and (3) exclusion of other ocular granulomatous diseases, such as ocular toxoplasmosis, sarcoidosis, ocular tuberculosis, and other fungal infection. Typical clinical features of OT include the presence of a peripheral granuloma (a focal, white peripheral nodule with pigmented scarring or traction retinal detachment), posterior pole granuloma (a focal, white nodule with or without posterior pole variable pigmentation), or nematode endophthalmitis (diffuse intraocular inflammation and serology results only positive for Toxocara) [5].
The indirect enzyme-linked immunosorbent assay (ELISA), which measures immunoglobulin G (IgG) antibody titers using the Toxocara canis larva antigen, is currently used for serologic diagnosis of OT [6]. However, a differential diagnosis of OT in patients with uveitis of unknown etiology is sometimes challenging, and the interpretation of the results obtained from the ELISA test is not always simple. Thus, the aim of this study was to evaluate the diagnostic value of an ELISA test for anti-Toxocara IgG for OT in patients with uveitis for which they visited a tertiary hospital in Korea.
All aspects of the research protocol were in compliance with the Declaration of Helsinki. The institutional review board of Seoul National University Bundang Hospital (SNUBH) approved this study. The medical records of 241 consecutive patients with active intraocular inflammation of unknown etiology who visited SNUBH between January 2010 and June 2012 were retrospectively reviewed. These patients were screened with serum anti-Toxocara IgG ELISA, and other laboratory studies were conducted to identify the cause of uveitis. A complete blood count, total serum IgE level, serum angiotensin converting enzyme level, rheumatoid factor, and human leukocyte antigen-B51 and B27 measurements were obtained. Other serologic tests were also performed including toxoplasma antibody test, fluorescent treponemal antibody absorption test, venereal disease research laboratory test, and interferon gamma release assay.
Radiologic examination involving plain radiographs of either the chest or the pelvis was conducted to rule out other causes of ocular inflammation, including granulomatous uveitis due to sarcoidosis and tuberculosis. Computed tomography scans of the abdomen and chest were performed in selected patients with abnormal findings on simple radiography. Slit lamp and fundus examinations, optical coherence tomography, and fluorescein angiography were also carried out, and these results were evaluated by two experienced retinal specialists (SJW and KHP). We diagnosed OT based on (1) typical clinical findings; (2) ancillary laboratory tests, such as total serum IgE level and eosinophil count; and (3) exclusion of other intraocular granulomatous disease. Three patients were excluded because differentiation between OT and ocular toxoplasmosis was not possible. Therefore, the final analyses included 238 total patients. The participants were questioned about generalized symptoms, including fever, night sweats, pulmonary or extrapulmonary symptoms, weight loss, and lower back pain; contact with dogs or cats; and consumption of raw meat.
We performed ELISA test, which measures IgG antibody titer specific to Toxocara canis larva crude antigen (TCLA). The cut-off value for TCLA ELISA was 0.250; a titer value greater than 0.250 was defined as a positive result [6].
Patients were divided into four groups with a primary focus on ocular inflammation: anterior uveitis, intermediate uveitis, panuveitis, and posterior uveitis. Clinical factors, such as age, sex, serum anti-Toxocara IgG titer, and anatomic type, were compared between patients with positive and negative toxocariasis ELISA results using the chi-square test or Student's t-test. The same tests were used to compare these characteristics between the patients diagnosed with OT and all others. Continuous variables are shown as mean ± standard deviation. Statistical analyses were conducted using SPSS ver. 22.0 (IBM Co., Armonk, NY, USA). A p-value less than 0.05 indicated statistical significance.
The clinical characteristics of the 238 patients, who included 148 male and 90 female patients, are presented in Table 1. The mean age of the patients was 46.2 ± 16.9 years (range, 6 to 98 years). Among the 238 patients, 71 (29.8%) were diagnosed with OT, and 80 (33.6%) had positive serum toxocariasis IgG ELISA results. Sixty-five patients (91.5%) with OT had positive toxocariasis ELISA results, and 152 of the 167 patients (91.0%) who had not been diagnosed with OT had negative toxocariasis ELISA results. The sensitivity and specificity of the ELISA test were 91.5% (65 / 71) and 91.0% (152 / 167), respectively. The positive predictive value was 81.3% (65 / 80).
The mean age was significantly different between patients with OT and non-OT uveitis (53.2 ± 13.5 vs. 43.2 ± 17.3 years, p < 0.001, Student's t-test). Patients with OT had higher serum anti-Toxocara IgG titer level than the non-OT uveitis patients (0.361 ± 0.106 vs. 0.096 ± 0.098, p < 0.001, Student's t-test) (Fig. 1). Among the OT patients, 32 (45.1%) had recently consumed raw meat (mostly raw cow liver), and six (8.5%) owned pets.
Anatomic involvement of uveitis showed statistically significant differences between patients with positive and negative toxocariasis ELISA results (p < 0.001, chi-square test). In the patient group with positive toxocariasis ELISA results, posterior uveitis was the most common (n = 38, 47.5%), and intermediate uveitis was the second most prevalent (n = 24, 30.0%). However, in the patient group with negative toxocariasis ELISA results, anterior uveitis was most common (n = 59, 37.3%), and posterior uveitis was the second most common (n = 48, 30.4%).
The participants were divided into four subgroups according to the anatomic types of uveitis, as shown in Table 2. The incidence of OT was 8.3% (6 / 72), 47.1% (24 / 51), 44.8% (39 / 87), and 7.1% (2 / 28) in anterior, intermediate, posterior, and panuveitis, respectively. The seropositivity was 18.1% (13 / 72), 47.1% (24 / 51), 43.7% (38 / 87), and 17.9% (5 / 28) in anterior, intermediate, posterior, and panuveitis, respectively. The sensitivity of the toxocariasis ELISA test was 16.7% (1 / 6), 95.8% (23 / 24), 89.8% (35 / 39), and 100.0% (2 / 2) in anterior, intermediate, posterior, and panuveitis, respectively. The specificity was 87.9% (58 / 66), 96.3% (26 / 27), 93.8% (45 / 48), and 88.5% (23 / 26), in anterior, intermediate, posterior, and panuveitis, respectively. The positive predictive value was 38.5% (5 / 13), 95.8% (23 / 24), 92.1% (23 / 24), and 40.0% (2 / 5) in anterior, intermediate, posterior, and panuveitis, respectively. The prevalence of OT and the toxocariasis ELISA results were also significantly different between the subgroups (p < 0.001, chi-square test) (Fig. 2A and 2B).
Six patients who were clinically diagnosed with OT had seronegative results on the toxocariasis ELISA. They presented with the typical clinical findings of OT and had serum toxocariasis IgG titers that were lower than the cut-off value (range, 0.189 to 0.239) but were still higher than those of the non-OT patients (mean, 0.096 ± 0.098) (Fig. 1).
Of 71 OT patients, 37 (52.1%) had a symptom duration less than 3 months, which indicates that they had an acute inflammatory condition. Thirty-four patients experienced ocular inflammation that had lasted longer than 3 months, or they had an unknown date of onset. The former group was classified as having acute OT, while the latter group had chronic OT. The two groups demonstrated no significant differences in terms of age, sex, laterality, seropositivity, or ELISA titer, although anatomical involvement and symptom duration were different. In the patient group with acute OT, posterior and intermediate uveitis were equally most common (n = 15, 40.5%), whereas posterior uveitis was predominant in the chronic OT group (n = 24, 70.6%), followed by intermediate uveitis (n = 9, 26.5%). The mean symptom duration of the chronic OT patients was longer than that of the acute OT patients (15.76 ± 20.58 vs. 0.76 ± 0.80 months, p < 0.005, Mann-Whitney U-test) (Table 3).
Thirty-four OT patients had been treated with albendazole, and follow-up ELISA tests were completed regularly for 30 patients (duration, 15.10 ± 20.76 weeks; range, 3.8 to 73.6 weeks). The serum anti-Toxocara IgG titer had decreased by 22.4% ± 40.8% (range, -64.0% to 98.8%) following albendazole treatment (0.382 ± 0.134 vs. 0.274 ± 0.136, p < 0.001, paired t-test) (Fig. 3).
This study showed that an ELISA test of serum anti-Toxocara IgG had remarkable value in the diagnosis of OT in terms of sensitivity (91.5%), specificity (91.0%), and positive predictive value (81.3%). In a subgroup analysis based on anatomical types, OT was more prevalent in intermediate and posterior uveitis, and the toxocariasis ELISA test also provided more confident results in these types.
Intraocular inflammation has many different causes and presents with various symptoms and clinical signs. Uveitis often results in visual disturbance, and vision can deteriorate when adequate treatment is delayed. Therefore, it is important to determine the origin of intraocular inflammation and promptly manage the underlying cause in treatable cases. OT is an etiological factor of infectious uveitis, but its clinical importance seems to be neglected and underestimated [78]. It has been reported that OT accounts for only 1% of posterior uveitis [9]. Other studies have demonstrated that the seroprevalence is up to 46% in adults and can reach 77.6% in children. In the United States, the overall prevalence varied from 4.6% to 30%, depending on the region and socioeconomic status [810]. In East Asia, OT usually occurs in adults; Yoshida et al. [11] reported that 89% of OT patients in Japan were older than 20 years. In the present study, the prevalence of OT in patients who had ocular inflammation was 29.8% (71 / 238), and this result is comparable with the results of another study conducted in South Korea, which reported a seroprevalence of Toxocara canis of 23.5% in patients with uveitis [12].
OT can be definitively diagnosed with a biopsy for direct confirmation of Toxocara infection. However, obtaining a biopsy specimen is risky and not always an option. Therefore, OT is mainly diagnosed using indirect and clinical evidence. It has been reported that the diagnostic value of the serum toxocariasis ELISA test is high in terms of sensitivity and specificity, making it a promising diagnostic tool [6]. In the present study, the sensitivity and specificity of the ELISA test for serum anti-Toxocara IgG were 91.5% and 91.0%, respectively. The positive predictive value was 81.3%.
Anatomic subgroup analysis revealed that OT was more prevalent in intermediate and posterior uveitis types. It was also shown that the positive predictive value was higher in intermediate uveitis and posterior uveitis types than in the other anatomical types. The sensitivity and specificity of the toxocariasis ELISA test were lower in patients with anterior uveitis. Overall, the toxocariasis ELISA test should be considered an essential laboratory test in patients with intraocular inflammation, especially in cases of posterior or intermediate uveitis.
Acute OT patients usually had higher anti-Toxocara IgG levels, and the current cut-off value (0.250) for the ELISA test for serum anti-Toxocara IgG seems to be acceptable in these cases. However, in chronic OT patients, there were some cases where the typical clinical findings led to a clinical diagnosis of OT even when the ELISA titer was relatively low. Of the six patients with intermediate IgG titer level, five had chronic inflammation, and only the remaining one patient was diagnosed with acute OT. Therefore, in chronic OT cases, it might be reasonable to designate a serum toxocariasis IgG titer cut-off value that is lower than the usual cut-off value.
There were no statistically significant differences in clinical characteristics, including ELISA titer, between the acute and chronic OT groups, although anatomical involvement of inflammation was difference (Table 3). The serum anti-Toxocara IgG titer significantly decreased following albendazole treatment. These results suggest that the toxocariasis ELISA test reflects the load of toxocariasis infection and would be helpful in evaluating treatment response and disease activity during follow-up of an individual patient. However, the toxocariasis ELISA results can be determined not only by the duration of inflammation, but also by other host factors, including the immune response to Toxocara larva. The toxocariasis ELISA result should be interpreted carefully because interpersonal variability in test results might influence the serum anti-Toxocara IgG titer.
Fifteen patients who showed positive ELISA results were not diagnosed with OT. In these cases, asymptomatic, non-ocular Toxocara infection, such as that in the liver, lung, or brain, could explain these results. The high frequency of a history of ingestion of raw meat and cow liver usually confirm asymptomatic infections.
The sensitivity and specificity of serum ELISA have both been previously reported as approximately 90%, respectively [13]. Here, we conducted a large-scale study to determine the diagnostic value of the serum anti-Toxocara IgG ELISA in Korean OT patients, which could be valuable for interpretation of assay results and for differential diagnosis. In addition, we used a TCLA ELISA kit developed by the Seoul National University College of Medicine. Jin et al. [6] reported that the sensitivity and specificity of the TCLA ELISA for human toxocariasis are 92.2% and 86.6%, respectively. The positive and negative predictive values of human toxocariasis have been reported as 78.7% and 97.8%, respectively [6]. Thus, the TCLA ELISA kit used in this study has been shown to be acceptable for serodiagnosis of human toxocariasis.
Our study had several limitations that require further consideration. This was a retrospective study, and there might have been a selection bias because SNUBH is a tertiary referral hospital, and the researchers are well-known experts in OT. In addition, OT was diagnosed clinically instead of using a definitive confirmation of Toxocara larval infection; therefore, the prevalence of OT could have been overestimated.
In conclusion, OT is a common cause of intraocular inflammation in Korea. Serum anti-Toxocara IgG ELISA can be very useful in the differential diagnosis of uveitis and also for evaluating the activity of the disease. Considering that OT is more prevalent in patients with intermediate and posterior uveitis and also that the positive predictive value of the anti-Toxocara IgG assay is high, a routine test for anti-Toxocara IgG is necessary for patients with these types of uveitis in Korea.
Figures and Tables
Fig. 1
Serum anti-Toxocara IgG titers in OT and non-OT uveitis patients. Patients with ocular toxocariasis (OT) had higher serum anti-Toxocara immunoglobulin G titer results than non-OT uveitis patients (0.361 ± 0.106 vs. 0.096 ± 0.098, p < 0.001). Six patients who were clinically diagnosed with OT had relatively high anti-Toxocara immunoglobulin G titer results but did not exceed the cut-off value (0.250).

Fig. 2
Patients were divided into four subgroups according to anatomic types of uveitis: anterior, intermediate, posterior, or panuveitis. The prevalence of ocular toxocariasis was 8.3% (6 / 72), 47.1% (24 / 51), 44.8% (39 / 87), and 7.1% (2 / 28), respectively, for anterior, intermediate, posterior, or panuveitis (A). The seropositivity was 18.1% (13 / 72), 47.1% (24 / 51), 43.7% (38 / 87), and 17.9% (5 / 28), respectively (B). The prevalence of ocular toxocariasis and enzyme-linked immunosorbent assay results for serum anti-Toxocara immunoglobulin G were significantly different between subgroups (p < 0.001). The p-values were obtained using the chi-square test.
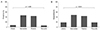
Fig. 3
The change of titer for serum anti-Toxocara immunoglobulin G before and after albendazole treatment (n = 30). ELISA = enzyme-linked immunosorbent assay.
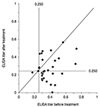
Table 1
Clinical characteristics of the included patients, comparisons between patients with positive and negative serum anti-Toxocara immunoglobulin G ELISA test results, and comparisons between patients with and without OT

Table 2
The ELISA test results for serum anti-Toxocara immunoglobulin G analyzed based on the anatomical types of uveitis

Acknowledgements
This study was supported by a grant (CCP-13-02-KIST) from the Convergence Commercialization Project of National Research Council of Science and Technology, Seoul, Korea.
References
1. Kwon SI, Lee JP, Park SP, et al. Ocular toxocariasis in Korea. Jpn J Ophthalmol. 2011; 55:143–147.
2. Despommier D. Toxocariasis: clinical aspects, epidemiology, medical ecology, and molecular aspects. Clin Microbiol Rev. 2003; 16:265–272.
3. Taylor MR. The epidemiology of ocular toxocariasis. J Helminthol. 2001; 75:109–118.
4. Ahn SJ, Ryoo NK, Woo SJ. Ocular toxocariasis: clinical features, diagnosis, treatment, and prevention. Asia Pac Allergy. 2014; 4:134–141.
5. Ahn SJ, Woo SJ, Jin Y, et al. Clinical features and course of ocular toxocariasis in adults. PLoS Negl Trop Dis. 2014; 8:e2938.
6. Jin Y, Shen C, Huh S, et al. Serodiagnosis of toxocariasis by ELISA using crude antigen of Toxocara canis larvae. Korean J Parasitol. 2013; 51:433–439.
7. Rubinsky-Elefant G, Hirata CE, Yamamoto JH, Ferreira MU. Human toxocariasis: diagnosis, worldwide seroprevalences and clinical expression of the systemic and ocular forms. Ann Trop Med Parasitol. 2010; 104:3–23.
8. Hotez PJ, Wilkins PP. Toxocariasis: America's most common neglected infection of poverty and a helminthiasis of global importance? PLoS Negl Trop Dis. 2009; 3:e400.
9. Stewart JM, Cubillan LD, Cunningham ET Jr. Prevalence, clinical features, and causes of vision loss among patients with ocular toxocariasis. Retina. 2005; 25:1005–1013.
10. de Visser L, Rothova A, de Boer JH, et al. Diagnosis of ocular toxocariasis by establishing intraocular antibody production. Am J Ophthalmol. 2008; 145:369–374.
11. Yoshida M, Shirao Y, Asai H, et al. A retrospective study of ocular toxocariasis in Japan: correlation with antibody prevalence and ophthalmological findings of patients with uveitis. J Helminthol. 1999; 73:357–361.
12. Lim SJ, Lee SE, Kim SH, et al. Prevalence of Toxoplasma gondii and Toxocara canis among patients with uveitis. Ocul Immunol Inflamm. 2014; 22:360–366.
13. Foster CS. Ocular toxocariasis. In : Foster CS, Vitale AT, editors. Diagnosis and treatment of uveitis. 2nd ed. New Delhi: JayPee Brothers Medical;2013. p. 611.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download