Abstract
Purpose
To evaluate the effects of posterior subtenon triamcinolone acetonide injection on refractory diabetic macular edema (DME) after intravitreal bevacizumab (IVB) injection failure.
Methods
Patients with DME and central subfield thickness (CST) >300 µm who did not respond to IVB injections were retrospectively included. Specifically, we enrolled patients who were diagnosed with refractory DME and who experienced an increase in CST after 1 to 2 IVB injections or no decrease after ≥3 consecutive IVB injections. One clinician injected 20 mg of triamcinolone acetonide into the posterior subtenon space. All patients received ophthalmic examinations at baseline and at 2, 4, and 6 months post-baseline. Examinations included Snellen visual acuity, intraocular pressure, and spectral-domain optical coherence tomography.
Results
Forty eyes of 34 patients were included. The average baseline CST was 476 µm. The average CST decreased to 368 µm at 2 months, 374 µm at 4 months, and 427 µm at 6 months (p < 0.001 for all results, Wilcoxon signed-rank test). The average intraocular pressure increased from 15.50 to 16.92 mmHg at 2 months but decreased to 16.30 mmHg at 4 months and 15.65 mmHg at 6 months. Logarithm of the minimum angle of resolution visual acuity improved from 0.56 to 0.50 at 2 months (p = 0.023), 0.50 at 4 months (p = 0.083), and 0.48 at 6 months (p = 0.133, Wilcoxon signed-rank test). No complications were detected.
The most common cause of visual disturbance in patients with diabetic retinopathy is diabetic macular edema (DME) [1]. Laser photocoagulation is the standard treatment for DME [23]. Antivascular endothelial growth factor (anti-VEGF) therapy was recently accepted as a first-line treatment for DME because numerous trials have reported its beneficial effects [45]. However, despite these dramatic outcomes, not all DME patients respond to anti-VEGF therapy. Nearly 50% of patients treated with ranibizumab in the RESTORE study [4] had an average central subfield thickness (CST) >275 µm at 12 months post-baseline. This means that nearly half of ranibizumab-treated patients still had thickened CST.
Triamcinolone acetonide (TA) has been used to treat DME [67], and intravitreal triamcinolone acetonide (IVTA) injection has shown efficacy against DME [78910]. However, use of IVTA injection is limited in clinical settings because it has been linked to the development of cataracts, elevated intraocular pressure (IOP), sterile pseudo endophthalmitis, and infectious endophthalmitis [71112]. Posterior subtenon triamcinolone acetonide (stTA) injection has been used to treat pseudophakic macular edema and uveitis [71314]. Contrastingly, some authors report that posterior stTA injection for DME treatment does not result in the previously described severe adverse events [71516].
In this study, we investigated the effects of stTA injection for refractory DME after 6 months of failed intravitreal bevacizumab (IVB) injection. We also evaluated potential adverse effects, including elevated IOP and cataract formation.
This retrospective, nonrandomized, interventional study was performed in accordance with the 1975 Helsinki Declaration and the 1983 revision. The study protocol was approved by the institutional review board of Asan Medical Center, Seoul, Korea.
The electronic medical records of patients who were diagnosed with DME between January 2011 and December 2012 at Asan Medical Center were reviewed. Patients with DME involving the fovea, a CST >300 µm, and who did not respond to IVB injection were included. We defined DME as refractory to IVB if either of the following conditions were met: (1) CST did not decrease by more than 30 µm after ≥3 consecutive IVB injections, or (2) CST increased after 1 to 2 IVB injections. Exclusion criteria were: (1) <18 years; (2) history of retinal vein occlusion, retinal arterial occlusion, uveitis, epiretinal membrane, or any chorioretinal disease other than diabetic retinopathy; (3) previous focal or grid laser treatment; (4) panretinal photocoagulation treatment <3 months before the first IVB injection; (5) previous IVTA or stTA treatment; (6) suspected glaucoma (with a high cup to disc ratio, >0.6) or diagnosis of glaucoma by a glaucoma specialist; and (7) any kind of ocular surgery, including cataract surgery, within the last 6 months.
Posterior subtenon injections were administered by a single retina specialist (SGJ), all using the same protocol. Patients were placed on a bed in the outpatient clinic and, after applying sterile draping, triamcinolone (20 mg/0.5 mL) was injected through the inferior fornix to the posterior subtenon space. Patients were prescribed Trusopt (dorzolamide hydrochloride ophthalmic solution; Merck, Kenilworth, NJ, USA) to control subsequent IOP. Patients were examined every 2 months after injection.
Each patient's best-corrected visual acuity (BCVA), IOP, and CST were evaluated on the day of stTA injection and again at 2, 4, and 6 months. At each visit, lens status was evaluated to determine if the posterior subcapsular cataract was more advanced than PII (according to the Lens Opacities Classification System III). BCVA was assessed using a Snellen visual acuity chart, and IOP was measured using Goldmann applanation tonometry. We prescribed Trusopt for prophylactic IOP control. Other related factors, such as duration of diabetes, glomerular filtration rate, and hemoglobin A1c (HbA1c), were also assessed.
CST was measured using spectral-domain optical coherence tomography (Spectralis; Heidelberg Engineering, Heidelberg, Germany). The average thickness of all points within the inner 1-mm-diameter circle was defined as the CST of the fovea and was based on the subfields used in the Early Treatment Diabetic Retinopathy Study. OCT images were obtained for all 25 cross-sectional lines that were 240 µm apart. In order to improve image quality, we used the automatic real-time technique. An eye-tracking system attached to an OCT machine was also used to obtain better images and precisely compare CST values between follow-up examinations. Macular edema patterns on OCT were classified according to the criteria previously reported by Otani et al. [17] and Shimura et al. [18], including sponge-like diffuse retinal thickening (SDRT), cystoid macular edema (CME), serous retinal detachment, and a combination of all three edema types (FULL).
All statistical tests were performed using SPSS ver. 15.0 (SPSS Inc., Chicago, IL, USA). Results between each follow-up period were compared using the paired t-test.
In total, 34 patients (40 eyes) met our inclusion criteria. Of these, 33 patients (36 eyes) received 2 months of follow-up examinations. Thirty-three patients received 4 months of follow-up examinations. One patient did not receive a spectral-domain optical coherence tomography examination. At 6 months, 31 patients (37 eyes) received all examinations. The baseline characteristics of the 34 patients are summarized in Table 1; the average patient age was 61.7 ± 9.19 years, and 21 of these patients were male. The mean baseline CST was 476 ± 153 µm (range, 300 to 988 µm). Mean CST decreased to 368 µm (p < 0.001, Wilcoxon signed-rank test) within 2 months of receiving stTA injection, then increased slightly to 374 µm. It subsequently resumed its decreasing trend from baseline at 4 months (p < 0.001, Wilcoxon signed rank test). CST increased to 427 µm by 6 months but was still lower than baseline (p = 0.046, Wilcoxon signed-rank test) (Fig. 1). Twenty-five eyes were diagnosed with SDRT, 12 eyes were diagnosed with CME, and three eyes were diagnosed with FULL. No cases were diagnosed as serous retinal detachment type. We did not identify any CST differences between the classified forms of macular edema at any of the time points. The effects of stTA, which were measured in terms of CST change at each follow-up OCT examination, did not indicate any differences between groups (data not shown).
The mean initial BCVA was 0.55 on the logMAR scale. The BCVA improved to 0.50 at 2 months after stTA injection, remained at 0.50 after 4 months, and finally decreased to 0.48 by the 6-month follow-up examination; only the value at 2 months represented a statistically significant change (p = 0.023, p = 0.083, and p = 0.133, respectively; Wilcoxon signed-rank test). Changes in visual acuity (VA) are presented in Table 2.
The changes in IOP are shown in Table 3. The average IOP changed significantly from 15.50 mmHg at baseline to 16.92 mmHg at 2 months (p = 0.040, paired t-test). However, IOP did not change significantly at 4 months (16.30 mmHg, p = 0.103) or at 6 months (15.65 mmHg, p = 0.732, paired t-test). Three eyes from 37 eyes were administered another IOP-lowering drug. No eyes had an IOP >21 mmHg during the entire follow-up period.
Among all 40 eyes, 27 were phakic and 13 were pseudophakic. We did not identify any cases of advanced cataract during the follow-up period (i.e., no eyes were >PII according to the Lens Opacities Classification System III classification). No other stTA-related complications were noted during the study period.
Due to their anti-inflammatory effects, inhibitory effects on VEGF synthesis, and role in reducing vascular permeability, corticosteroids are an important component of DME treatment [1920212223]. Among the many corticosteroids available, TA has been adopted to treat DME because of its anti-angiogenic, anti-inflammatory, and blood retinal barrier-stabilizing effects [2425]. TA can be delivered via stTA or intravitreal injection. The effects of IVTA on DME have been discussed in many studies [68]. IVTA also has some limitations, including elevated IOP cataract progression, pseudo endophthalmitis, and infectious endophthalmitis [711122627].
Ozdek et al. [28] compared the effects of IVTA and stTA. They reported that both stTA and IVTA significantly affect DME treatment, especially in the short-term, and that, although the effects were more pronounced in the IVT group, stTA also seemed to be a safe and effective technique for treating DME [28]. Bakri and Kaiser [7] reported that the therapeutic effects of stTA on DME are refractory to laser photocoagulation. Recently, many anti-VEGF drugs have been widely used to treat DME. However, as discussed in the introduction, a large portion of DME cases does not respond to anti-VEGF. To the best of our knowledge, no previous reports have analyzed the effects of stTA on DME refractory to IVB injection.
In this study, the effect of stTA did not appear to last for 6 months. Ozdek et al. [28] found that the effects of stTA start to diminish after 3 months. Fig. 2A-2H presents two such cases: the first case demonstrated representative responses to stTA. In the second case, CME completely disappeared after only one stTA injection.
We administered IVB injection to three eyes that had increased CST after the 2-month stTA injection. By the 4-month follow-up appointment, CST had decreased in two of the eyes, but one eye still had heightened CST. Of the two eyes with decreased CST, only one had steadily decreasing CST by the 6-month visit. CST had decreased by 129 µm since the 4-month visit. This decrease might have been due to delayed stTA effects or a combination of effects due to the 2-month IVB injection administration.
Regarding DME type and treatment response, Roh et al. [29] reported that patients whose OCT showed signs of CME were more likely to have greater improvement in terms of VA and macular thickness following IVB injection. On the other hand, Kim et al. [30] and Shimura et al. [18] concluded that SDRT yielded a better response to IVB injection. Shimura et al. [31] also found that IVTA was a more effective treatment for patients with CME, while IVTA was less effective for patients with serous retinal detachment. In this study, we found no differences in the responsiveness to stTA that were associated with DME type. Because SDRT eyes that demonstrated a good response to IVB injection were not included in this study, our results are not comparable to previous findings on IVTA [31].
VA improved after 2 months but did not show improvements from baseline at 4 or 6 months. Although improvements in VA were not statistically significant at the 4- or 6-month visit, VA did improve as CST decreased among our patients. Santos et al. [32] also reported correlations between decreased CST and improved VA. Long-standing DME that does not respond to treatment might lead to photoreceptor damage and visual impairment [33]. Therefore, preventing long-standing DME is important for avoiding severe visual loss. At 6 months, 16 eyes in the present study had increased VA and 10 eyes had decreased VA. Eyes with increased VA presented with a thinner CST at baseline as well as at 2, 4, and 6 months compared to eyes with decreased VA. Eyes with increased VA had a thinner CST at 2, 4, and 6 months than eyes with decreased VA. However, these results were not statistically significant. VA improved with stTA but was unrelated to glomerular filtration rate or duration of diabetes. Eyes with improved VA also had higher HbA1c level (p = 0.049). These results are consistent with those reported by Matsuda et al. [34].
Park et al. [35] found a similar pharmacokinetic result in their animal model, indicating similar conclusions regarding the duration effect of stTA. Park et al. [35] reported that the effect of 40 mg stTA was observed for at least 3 months in rabbit eyes. In our study, the effect of stTA decreased between the 2- and 4-month visit.
CST changes were unrelated to duration of diabetes and glomerular filtration rate. Eyes with a CST decrease greater than 30 µm at 6 months, in comparison to baseline, had lower HbA1c levels; however, this result was not statistically significant (p = 0.160).
For most patients, IOP increased slightly at 2 months, but it returned to baseline level in most of our cases. Three eyes were prescribed other IOP-lowering drugs at 2 months, all of which maintained the initial IOP through 4 months of follow-up. Most of the eyes, except these three, showed stable IOP across the 6-month follow-up period without the use IOP-lowering drugs. Bakri and Kaiser [7] reported slightly increased IOP at 3 months that was restored at 6 months in their 12-month follow-up study on the effects of stTA on DME refractory to laser treatment. Choi et al. [16] reported that IVTA and stTA had similar effects on DME, but that IVTA increased IOP after 3 months. Cellini et al. [36] and Qi et al. [24] also found similar results in their 6-month follow-up study. Ozdek et al. [28] found that 8.2% of the stTA patients showed a significant increase in IOP (>21 mmHg), and 24.3% of patients in the IVTA group had a significant increase. Due to the administration of high-dose steroids in a study by Jonas et al. [8], up to 50% of patients who received IVTA had elevated IOP. stTA was also found to be associated with cataract progression, central retinal vein occlusion, inadvertent injection into the choroidal or retinal circulation, perforation of the globe, and central retinal artery occlusion [283738]. We did not find any record of cataract progression in patient's medical records. Bakri and Kaiser [7] and Cellini et al. [36] reported no development of cataract progression in their stTA-treated patients. We also did not note any other complications known to be related to stTA, such as perforation, retinal vein occlusion, or inadvertent injection.
This study has several limitations. The analyses were retrospectively performed without a control group. VA was examined using the Snellen VA chart instead of the Early Treatment Diabetic Retinopathy Study chart. The administration of dorzolamide eye drops might have contributed to the observed edema decreases. Even though there have been no reports on the effects of dorzolamide on DME, the effects of other types of macular edema, such as CME, on retinitis pigmentosa patients have been reported [3940]. Elevated IOP after stTA might also be obscured by dorzolamide. Six months of follow-up examinations were insufficient to observe long-term effects or complications of longer follow-up periods could reveal additional findings.
In this study, we demonstrated an effect of stTA on DME refractory to bevacizumab for the first time, and we also showed that stTA was associated with a lower rate of adverse events than previous study, like cataract progression or elevated IOP during the follow up period.
In conclusion, stTA is an effective, safe, and affordable treatment for reducing CST in DME refractory to IVB injection.
Figures and Tables
 | Fig. 1Changes in average central subfield thickness (CST) following posterior subtenon triamcinolone injection: mean CST had decreased by the 2-month visit and was maintained through the 4-month visit. However, mean CST had increased by the 6-month visit. The p-values are indicated by bars and were estimated using the Wilcoxon signed-rank test and represent comparisons to the baseline CST values. |
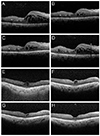 | Fig. 2Two cases are illustrated. (A-D) The first case demonstrated a typical response to posterior subtenon triamcinolone acetonide (stTA) injection. The patient received 7 consecutive intravitreal bevacizumab (IVB) injections before receiving stTA. (A) Central subfield thickness (CST) was 467 µm after receiving 7 IVB injections, and visual acuity (VA) was 0.32 according to the Snellen visual acuity chart. (B) Two months later, CST decreased to 346 µm and VA improved to 0.4. (C) Four months later, CST increased slightly to 363 µm and VA decreased slightly to 0.32. (D) Six months later, CST increased to 424 µm and VA increased to 0.5. (E-H) Cystoid macular edema (CME) completely disappeared in the second case after only one stTA injection with no other treatments. This patient received 3 serial IVB injections. (E) CME did not respond, and VA was 0.63 after 3 IVB injections. (F,G) At 2 and 6 months later, CME decreased but VA improved to 0.8. (H) CME completely disappeared by the 9-month visit, and CME did not recur until the most recent 12-month visit. By then, VA had improved to 1.0. |
References
1. Klein R, Klein BE, Moss SE, et al. The Wisconsin epidemiologic study of diabetic retinopathy. IV. Diabetic macular edema. Ophthalmology. 1984; 91:1464–1474.
2. Early Treatment Diabetic Retinopathy Study research group. Photocoagulation for diabetic macular edema: early Treatment Diabetic Retinopathy Study report number 1. Arch Ophthalmol. 1985; 103:1796–1806.
3. Thomas BJ, Shienbaum G, Boyer DS, Flynn HW Jr. Evolving strategies in the management of diabetic macular edema: clinical trials and current management. Can J Ophthalmol. 2013; 48:22–30.
4. Mitchell P, Bandello F, Schmidt-Erfurth U, et al. The RESTORE study: ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema. Ophthalmology. 2011; 118:615–625.
5. Brown DM, Nguyen QD, Marcus DM, et al. Long-term outcomes of ranibizumab therapy for diabetic macular edema. The 36-month results from two phase III trials: RISE and RIDE. Ophthalmology. 2013; 120:2013–2022.
6. Martidis A, Duker JS, Greenberg PB, et al. Intravitreal triamcinolone for refractory diabetic macular edema. Ophthalmology. 2002; 109:920–927.
7. Bakri SJ, Kaiser PK. Posterior subtenon triamcinolone acetonide for refractory diabetic macular edema. Am J Ophthalmol. 2005; 139:290–294.
8. Jonas JB, Kreissig I, Sofker A, Degenring RF. Intravitreal injection of triamcinolone for diffuse diabetic macular edema. Arch Ophthalmol. 2003; 121:57–61.
9. Sutter FK, Simpson JM, Gillies MC. Intravitreal triamcinolone for diabetic macular edema that persists after laser treatment: three-month efficacy and safety results of a prospective, randomized, double-masked, placebo-controlled clinical trial. Ophthalmology. 2004; 111:2044–2049.
10. Yilmaz T, Weaver CD, Gallagher MJ, et al. Intravitreal triamcinolone acetonide injection for treatment of refractory diabetic macular edema: a systematic review. Ophthalmology. 2009; 116:902–911.
11. Diabetic Retinopathy Clinical Research Network. A randomized trial comparing intravitreal triamcinolone acetonide and focal/grid photocoagulation for diabetic macular edema. Ophthalmology. 2008; 115:1447–1459.
12. Elman MJ, Bressler NM, Qin H, et al. Expanded 2-year follow-up of ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2011; 118:609–614.
13. Thach AB, Dugel PU, Flindall RJ, et al. A comparison of retrobulbar versus sub-Tenon's corticosteroid therapy for cystoid macular edema refractory to topical medications. Ophthalmology. 1997; 104:2003–2008.
14. Jennings T, Rusin MM, Tessler HH, Cunha-Vaz JG. Posterior sub-Tenon's injections of corticosteroids in uveitis patients with cystoid macular edema. Jpn J Ophthalmol. 1988; 32:385–391.
15. Toda J, Fukushima H, Kato S. Injection of triamcinolone acetonide into the posterior sub-tenon capsule for treatment of diabetic macular edema. Retina. 2007; 27:764–769.
16. Choi YJ, Oh IK, Oh JR, Huh K. Intravitreal versus posterior subtenon injection of triamcinolone acetonide for diabetic macular edema. Korean J Ophthalmol. 2006; 20:205–209.
17. Otani T, Kishi S, Maruyama Y. Patterns of diabetic macular edema with optical coherence tomography. Am J Ophthalmol. 1999; 127:688–693.
18. Shimura M, Yasuda K, Yasuda M, Nakazawa T. Visual outcome after intravitreal bevacizumab depends on the optical coherence tomographic patterns of patients with diffuse diabetic macular edema. Retina. 2013; 33:740–747.
19. Dutra Medeiros M, Postorino M, Navarro R, et al. Dexamethasone intravitreal implant for treatment of patients with persistent diabetic macular edema. Ophthalmologica. 2014; 231:141–146.
20. Abraham SM, Lawrence T, Kleiman A, et al. Antiinflammatory effects of dexamethasone are partly dependent on induction of dual specificity phosphatase 1. J Exp Med. 2006; 203:1883–1889.
21. Barnes PJ. Corticosteroid effects on cell signalling. Eur Respir J. 2006; 27:413–426.
22. Saklatvala J. Glucocorticoids: do we know how they work? Arthritis Res. 2002; 4:146–150.
23. Walker BR. Glucocorticoids and cardiovascular disease. Eur J Endocrinol. 2007; 157:545–559.
24. Qi HP, Bi S, Wei SQ, et al. Intravitreal versus subtenon triamcinolone acetonide injection for diabetic macular edema: a systematic review and meta-analysis. Curr Eye Res. 2012; 37:1136–1147.
25. Ciulla TA, Walker JD, Fong DS, Criswell MH. Corticosteroids in posterior segment disease: an update on new delivery systems and new indications. Curr Opin Ophthalmol. 2004; 15:211–220.
26. Roth DB, Chieh J, Spirn MJ, et al. Noninfectious endophthalmitis associated with intravitreal triamcinolone injection. Arch Ophthalmol. 2003; 121:1279–1282.
27. Moshfeghi DM, Kaiser PK, Scott IU, et al. Acute endophthalmitis following intravitreal triamcinolone acetonide injection. Am J Ophthalmol. 2003; 136:791–796.
28. Ozdek S, Bahceci UA, Gurelik G, Hasanreisoglu B. Posterior subtenon and intravitreal triamcinolone acetonide for diabetic macular edema. J Diabetes Complications. 2006; 20:246–251.
29. Roh MI, Kim JH, Kwon OW. Features of optical coherence tomography are predictive of visual outcomes after intravitreal bevacizumab injection for diabetic macular edema. Ophthalmologica. 2010; 224:374–380.
30. Kim M, Lee P, Kim Y, et al. Effect of intravitreal bevacizumab based on optical coherence tomography patterns of diabetic macular edema. Ophthalmologica. 2011; 226:138–144.
31. Shimura M, Yasuda K, Nakazawa T, et al. Visual outcome after intravitreal triamcinolone acetonide depends on optical coherence tomographic patterns in patients with diffuse diabetic macular edema. Retina. 2011; 31:748–754.
32. Santos AR, Gomes SC, Figueira J, et al. Degree of decrease in central retinal thickness predicts visual acuity response to intravitreal ranibizumab in diabetic macular edema. Ophthalmologica. 2014; 231:16–22.
33. Channa R, Sophie R, Khwaja AA, et al. Factors affecting visual outcomes in patients with diabetic macular edema treated with ranibizumab. Eye (Lond). 2014; 28:269–278.
34. Matsuda S, Tam T, Singh RP, et al. The impact of metabolic parameters on clinical response to VEGF inhibitors for diabetic macular edema. J Diabetes Complications. 2014; 28:166–170.
35. Park HJ, Lee JE, Kim SI, et al. Intravitreal pharmacokinetics after posterior subtenon triamcinolone acetonide injection in vitrectomized rabbit eyes. Retina. 2014; 34:801–806.
36. Cellini M, Pazzaglia A, Zamparini E, et al. Intravitreal vs. subtenon triamcinolone acetonide for the treatment of diabetic cystoid macular edema. BMC Ophthalmol. 2008; 8:5.
37. Helm CJ, Holland GN. The effects of posterior subtenon injection of triamcinolone acetonide in patients with intermediate uveitis. Am J Ophthalmol. 1995; 120:55–64.
38. Mueller AJ, Jian G, Banker AS, et al. The effect of deep posterior subtenon injection of corticosteroids on intraocular pressure. Am J Ophthalmol. 1998; 125:158–163.
39. Grover S, Apushkin MA, Fishman GA. Topical dorzolamide for the treatment of cystoid macular edema in patients with retinitis pigmentosa. Am J Ophthalmol. 2006; 141:850–858.
40. Ikeda Y, Hisatomi T, Yoshida N, et al. The clinical efficacy of a topical dorzolamide in the management of cystoid macular edema in patients with retinitis pigmentosa. Graefes Arch Clin Exp Ophthalmol. 2012; 250:809–814.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download