Abstract
Purpose
Methods
Results
Figures and Tables
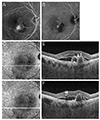 | Fig. 1Representative angiography and optical coherence tomography images in an eye diagnosed with polypoidal choroidal vasculopathy. (A) Fluorescein angiography. (B) Indocyanine green angiography. (C,D) Infrared and (E,F) optical coherence tomography. Multiple retinal pigment epithelial detachment (RPED) (E), sharp RPED peak (E, arrowhead), RPED notch (E, thick arrow), round hyporeflective area representing the polyp lumen (E, thin arrow), and intraretinal hard exudate (F, double arrows) were noted on optical coherence tomography images. |
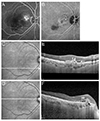 | Fig. 2Representative angiography and optical coherence tomography images in an eye diagnosed with polypoidal choroidal vasculopathy. (A) Fluorescein angiography. (B) Indocyanine green angiography. (C,D) Infrared and (E,F) optical coherence tomography. Multiple retinal pigment epithelial detachment (RPED) (E), sharp RPED peak (E, arrowhead), RPED notch (E, thick arrow), round hyporeflective area representing the polyp lumen (F, thin black arrow), and intraretinal hard exudate (F, double arrows) were noted on optical coherence tomography images. |
 | Fig. 3Changes in the logarithm of minimal angle of resolution (logMAR) best-corrected visual acuity (BCVA) in eyes diagnosed with polypoidal choroidal vasculopathy that underwent three consecutive monthly ranibizumab injections as an initial treatment. The closed circles (solid line) indicate the outcome in 111 indocyanine-green angiography-confirmed cases of polypoidal choroidal vasculopathy; the closed squares (dashed line) represent the outcome in 107 eyes diagnosed with the method suggested by De Salvo et al. [7]; the closed triangles (dotted line) suggest the outcome in 113 eyes diagnosed with a modified method, including the choroidal thickness criteria. |
Table 2
Distribution of OCT features in eyes exhibiting two or less features

OCT = optical coherence tomography; RPED = retinal pigment epithelial detachment; X = absence of OCT features; O = presence of OCT features; ICGA = indocyanine green angiography; PCV = polypoidal choroidal vasculopathy; AMD = age-related macular degeneration.
*Number of eyes exhibiting the OCT features presented in the same column.
Table 3
Distribution of OCT features in eyes exhibiting three or more features

OCT = optical coherence tomography; RPED = retinal pigment epithelial detachment; O = presence of OCT features; X = absence of OCT features; ICGA = indocyanine green angiography; PCV = polypoidal choroidal vasculopathy; AMD = age-related macular degeneration.
*Number of eyes exhibiting the OCT features presented in the same column.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download